Mars Actually "wetter" Than Antarctica Seasonally. |

  |
Mars Actually "wetter" Than Antarctica Seasonally. |
| Guest_RGClark_* |
 Dec 10 2005, 01:17 PM Dec 10 2005, 01:17 PM
Post
#1
|
|
Guests |
You frequently hear said Mars is much drier than any place on Earth as an indication of the difficulty of having liquid water on Mars, as in this news release:
Deep down, Mars harbors a lot of ice Frozen water may even be drinkable, scientists say. "Mars is extremely dry, drier than any (place) we have on Earth," said Gerhard Neukum, a German scientist who has analyzed stereo images of the Martian surface recorded by the European Space Agency's Mars Express satellite that began orbiting the fourth planet from the Sun in late 2003." http://www.sfgate.com/cgi-bin/article.cgi?...L&feed=rss.news This terminology "drier" refers to the amount of water vapor in its atmosphere. However, during southern Winter, Antarctica can actually have less atmospheric water vapor than the highest concentrations of water vapor on Mars: South Pole Transmissivity Plots. "Because of its high altitude, low water vapor column, and low temperatures, Antarctica may contain some of the driest and thus best sites for infrared, submillimeter, and millimeter astronomy [Bally, 1989; Harper, 1989; Chamberlin and Bally, 1996]. Potential sites on the Antarctic plateau vary in elevation from nearly 3000~m to over 4000~m. The center of the plateau is in a permanent high-pressure zone where air is descending from high altitudes. Temperatures at the south pole range from 200~K to 260~K [Chamberlin and Bally, 1994]. Measurements of the precipitable water vapor column [e.g., Smythe and Jackson, 1977; Burova et al., 1986] show that the water column can be as low as 50 microns in the austral winter, and is rarely above 1~mm." http://casa.colorado.edu/~bally/AT/cara.html The term "precipitable microns" means the thickness of liquid water you would have if the entire water vapor content in a column were condensed to liquid. Mars can have water vapor content above 100 precipitable microns over the northern pole in Summer, though the measurements close to the equator can be a as low as 10 precipitable microns. However, I believe there are some locations even near the equator on Mars that are far above the 10 micron level. For instance Noctis Labyrinthus frequently shows dense low lying clouds/fogs that give the appearance of precipitation carrying clouds on Earth: Noctis Labyrinthus 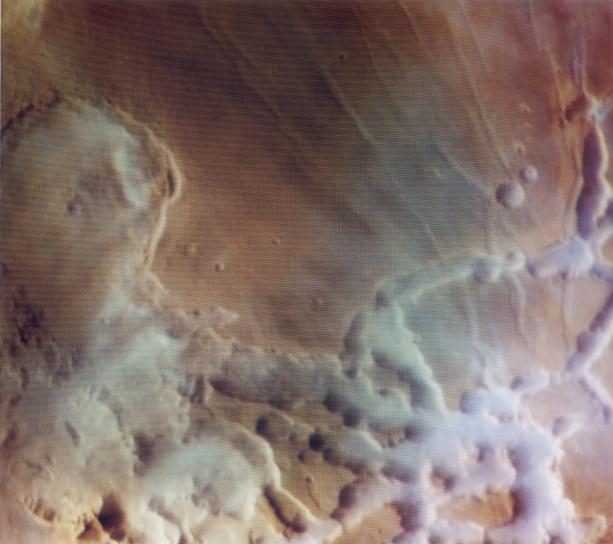 An even better image of these dense clouds/fogs is given in this report on Mars Express: Adsorption water driven processes on Mars. D. Möhlmann http://sci.esa.int/science-e/www/object/do...fobjectid=36779 [pdf file]  The mistaken idea that Mars' atmosphere always contains many times less water vapor than *anywhere* on Earth is a big reason why many Mars scientists discount the possibility of liquid water on the surface of Mars. Therefore I think it is important that the actual state of affairs is more well known among Mars scientists. Key also then is that there are isolated spots in Antarctica that still remain liquid even during the period of Antarctic winter when the atmospheric water vapor reaches these extremely low values. One such spot is Don Juan Pond: "H. This is a picture of Don Juan Pond, the only unfrozen body of water on the entire continent of Antarctica. It's located in the Wright Valley of the Dry Valleys and the "water" in the pond is an almost-saturated solution of calcium chloride. Don Juan Pond is so salty that it doesn't ever freeze, even in the dead of the Antarctic winter."  Other photos of Don Juan Pond located here: SIUC Microbiologists in Antarctica! http://www.science.siu.edu/microbiology/An.../Antarctic.html Note that such spots in Antarctica are so isolated that they wouldn't have been known from orbital imagery alone. Don Juan Pond was spotted from helicopter surveys and subsequentely investigated on foot. It may take such similar very low altitude investigation of Mars, perhaps only hundreds of feet altitude, before they are discovered there as well. My suggestion for where such surveys should start would be Noctis Labyrinthus. Bob Clark |
|
|
|
 Dec 11 2005, 09:16 PM Dec 11 2005, 09:16 PM
Post
#2
|
|
 Member    Group: Members Posts: 259 Joined: 23-January 05 From: Seattle, WA Member No.: 156 |
Bob - thanks for that bit about the precipitable water column. Yet another case where Martian environmental conditions overlap Earth's, even if only at the margins.
As far as brines go, while it's possible to get mixtures that will stay liquid at least as low as 213K (222K for CaCl2), that's pretty much assuming 1 bar pressure, isn't it? What happens if you lower the pressure on your brine to ~6-12mbar? |
|
|
|
| Guest_RGClark_* |
 Dec 13 2005, 01:20 PM Dec 13 2005, 01:20 PM
Post
#3
|
|
Guests |
Here's another version of the Viking Noctis image. The coloration is different and in this version, the valleys and peaks have the correct orientation:
Clouds in Noctis Labyrinthis. "This image shows early morning fog in the Noctis Labyrinthis, at the westernmost end of Valles Marineris. This fog, which is probably composed of water ice, is confined primarily to the low-lying troughs, but occasionally extends over the adjacent plateau. The region shown is about 300 kilometers (186 miles) across." http://www.solarviews.com/cap/mars/noctis.htm 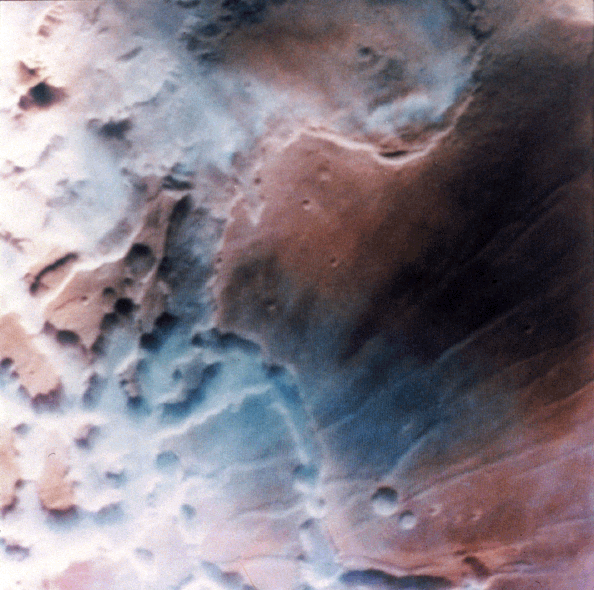 - Bob Clark |
|
|
|
| Guest_RGClark_* |
 Dec 13 2005, 01:45 PM Dec 13 2005, 01:45 PM
Post
#4
|
|
Guests |
QUOTE (Gsnorgathon @ Dec 11 2005, 09:16 PM) Bob - thanks for that bit about the precipitable water column. Yet another case where Martian environmental conditions overlap Earth's, even if only at the margins. As far as brines go, while it's possible to get mixtures that will stay liquid at least as low as 213K (222K for CaCl2), that's pretty much assuming 1 bar pressure, isn't it? What happens if you lower the pressure on your brine to ~6-12mbar? Good question. A detailed paper that discusses this question is by Haberle et.al.: On the possibility of liquid water on present-day Mars. Haberle, Mckay, Schaeffer, Cabrol, Grin, Zent, and Quinn. Journal of Geophysical Research, no. E10, p. 23,317-23,326, Oct. 25, 2001 It mentions that not only is the freezing point depressed by the addition of salts but so also is the equilibrium vapor pressure. That is, the required pressure for the water to stay liquid will be reduced: "We now examine the effect of dissolved salts on the potential for melting. Pure water is unlikely on Mars since salts are believed to be a significant component of the Martian soil [Clark and Van Hart, 1981]. The presence of salts will lower the melting point and reduce the equilibrium vapor pressure of the solution. An example of the effect of a NaCl brine on the potential for melting is shown in Figure 7. In this example, the eutectic point is 251 K and the equilibrium vapor pressure of the solution at that temperature is 1.23 mbar. Clearly, the presence of salts greatly expands the regions where melting could occur and increases the total time such conditions might exist. In this particular example, virtually the entire planet (except the polar regions) experiences conditions favorable for melting at some point during the year, including the Tharsis plateau. ... "For the NaCl brine mentioned above, boiling would not occur at its eutectic anywhere on Mars since the surface pressure never falls below 2.60 mbar anywhere on the planet during the year." On the possibility of liquid water on present-day Mars, p. 23,321-23,322 Bob Clark |
|
|
|
  |

|
Lo-Fi Version | Time is now: 28th April 2024 - 05:45 PM |
|
RULES AND GUIDELINES Please read the Forum Rules and Guidelines before posting. IMAGE COPYRIGHT |
OPINIONS AND MODERATION Opinions expressed on UnmannedSpaceflight.com are those of the individual posters and do not necessarily reflect the opinions of UnmannedSpaceflight.com or The Planetary Society. The all-volunteer UnmannedSpaceflight.com moderation team is wholly independent of The Planetary Society. The Planetary Society has no influence over decisions made by the UnmannedSpaceflight.com moderators. |
SUPPORT THE FORUM Unmannedspaceflight.com is funded by the Planetary Society. Please consider supporting our work and many other projects by donating to the Society or becoming a member. |

|