Printable Version of Topic
Click here to view this topic in its original format
Unmanned Spaceflight.com _ Phoenix _ Water Ice Confirmed!
Posted by: ugordan Jun 19 2008, 07:10 PM
Here's a flicker between sol 21 and 24 showing change (or, rather, lack of):
http://i108.photobucket.com/albums/n15/ugordan/sol21_24_flickr.gif
Ignore the color of the brightest part of the white stuff, it's overexposed. The small white chunk in sol 21 image appears to disappear in sol 24 (inset).
Posted by: akuo Jun 19 2008, 07:27 PM
Are you referring to the chunk that appeared on sol 19 and the was smeared into smaller bits on sol 20? Hard to say in the shadow, but those chunks may have indeed disappeared.
Posted by: ugordan Jun 19 2008, 07:28 PM
Yes, that one. It's in the shadow in sol 24 image, but other darker bits are subtly visible and yet this one isn't.
Posted by: Juramike Jun 19 2008, 08:14 PM
Nice animation!
There is another tiny chunk in the far right of the images (below the furthest right white streak) that is in full sunlight in both images that went away in the second image of the sequence.
-Mike
Posted by: Tomek Jun 19 2008, 08:51 PM
yes exactly
hortonheardawho from marsroverblog.com found this for sure now .
IT is ice
http://www.flickr.com/photos/hortonheardawho/2592881091/sizes/o/
Posted by: slinted Jun 19 2008, 09:09 PM
Here's my take on the sol 20 - sol 24 changes using the 5 filter (R1ABC2) set from each for comparison. Ugordon's warnings about the brightest features absolutely apply. There might be legitimate changes, but they might also just be overexposure / time-of-day / stretching differences.
The "bright stuff" under the shadow I feel much more confident about: it's there on sol 20, gone by sol 24.
http://www.lyle.org/~markoff/collections/dodo20_24comp_R1ABC2.gif
Posted by: Tomek Jun 19 2008, 09:20 PM
good point to obserwation is in this place also
http://www.flickr.com/photos/hortonheardawho/2592881091/sizes/o/
Posted by: fredk Jun 19 2008, 09:25 PM
Beautiful stuff, guys. I agree about the disappearing light bits in the shadow. But the lighting in the latest gifs (slinted, horton) is very similar on sols 20 and 24, as you can see by the shadows. We are again clearly seeing a darkening over time of the larger exposed substrate areas.
How does this sound: White substrate is mostly white ice, plus some dark dust/sand impurities. As the ice sublimates, the impurities are left behind. Eventually, the surface of the substrate is essentially completely covered by the dark impurities. That stage has almost been reached on the leftmost large exposed substrate area - there's very little white left by sol 24.
Posted by: elakdawalla Jun 19 2008, 10:44 PM
Awesome animation, slinted. That went straight to the blog. ![]()
--Emily
Posted by: belleraphon1 Jun 20 2008, 12:00 AM
slinted and all...
awesome indeed ... Peter Smith agrees...
http://phoenix.lpl.arizona.edu/06_19_pr.php
MUST BE ICE!!!!
Craig
Posted by: mars loon Jun 20 2008, 01:08 AM
MUST BE ICE!!!!
"It must be ice," said Phoenix Principal Investigator Peter Smith of the University of Arizona, Tucson. "These little clumps completely disappearing over the course of a few days, that is perfect evidence that it's ice. There had been some question whether the bright material was salt. Salt can't do that."
There is also an animation at the UA Phoenix page clearly showing the changes at lower left of Dodo-Goldilocks. Images from Sols 20 and 24 (June 15 and 18, 2008). "These images show sublimation of ice in the trench informally called "Dodo-Goldilocks" over the course of four days".
Pay Dirt !!
ken
Posted by: fredk Jun 20 2008, 01:09 AM
So as far as those disappearing chunks, how did they sublimate so fast? I thought we were talking microns per day of sublimation...
Posted by: Reed Jun 20 2008, 01:54 AM
That was for ice attached to a large body. A relatively small piece sitting on regolith would behave quite differently.
It also crosses my mind that those "chunks" might be more like shavings than solid blocks.
Posted by: Juramike Jun 20 2008, 02:33 AM
I so could not resist this! ![]()
http://www.youtube.com/watch?v=Vp-is6S_b_g
EDIT: poignant lyric for today:
"If there was a problem yo I'll solve it
Check out the hook while my DJ revolves it "
Posted by: stewjack Jun 20 2008, 03:10 AM
http://phoenix.lpl.arizona.edu/
NASA and the University of Arizona, Tucson, will hold a media teleconference at 10 a.m. PDT (1 p.m. EDT) (5 p.m. UTC) on Friday, June 20, to report on the latest news from NASA's Phoenix Mars Lander mission.
I wonder what the major topic will be? ![]()
Jack
Note This conference is only posted on the Arizona web page as of 11:00 p.m. EDT. I hope it will be announced on NASA's audio streaming page soon
http://www.nasa.gov/news/media/newsaudio/index.html
http://www.nasa.gov/news/media/newsaudio/index.htm
Posted by: glennwsmith Jun 20 2008, 04:19 AM
Whoa! I've just read an AP summary of the press conference in which it is stated that, yes, it IS ice under the thin, insulating layer of Martian dust. And I'm going to go out on a limb and agree with the exogeologists who have been surmising that this is not just a polar region phenomenon, but global, ie, Mars may have as much water. relatively speaking, as Earth, only it is locked away as ice under a relatively thin layer of Martian regolith. And thus volcanic activity can unleash the torrents of water in evidence all over the planet. And this perhaps also accounts for the "seepages" that we see in the sides of many craters. And thus it is likely as well that there are pockets -- perhaps huge pockets -- of liquid water at many, many places under the surface.
Posted by: Bobby Jun 20 2008, 04:58 AM
Ice on Mars! Now you see it, now you donít
Scientists say they know white stuff was frozen water because it vanished
http://www.msnbc.msn.com/id/25274243/
Posted by: elakdawalla Jun 20 2008, 05:12 AM
The http://news.yahoo.com/s/ap/20080620/ap_on_sc/phoenix_mars had at least one quote that was copied verbatim from the press release; the press conference hasn't happened yet. It does contain some news from Barry Goldstein saying that they now understand the sol 22 anomaly and have developed a software patch. Glennwsmith, there probably is lots of ice underneath the surface but it is not as close to the surface elsewhere on Mars (where it is warmer) as it is near the poles.
I hope to be able to listen in to tomorrow's press conference but I've got the baby on Fridays and I don't know what her plans are for me yet...
--Emily
Posted by: deglr6328 Jun 20 2008, 05:29 AM
How do we know this is water and not CO2 ice already? We all suspect this of course but the media is reporting it as fact. TEGA hasn't found any water yet so is there some IR spectral measurement I don't know about?
Posted by: elakdawalla Jun 20 2008, 05:40 AM
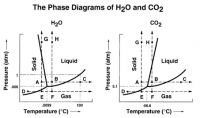
Too low pressure and high temperature for CO2 ice. We know the temperature at the landing site -- on sol 22, for instance, it was a high of -22 and a low of -80. And the pressure is very low, on the order of 8 millibars. If you look at a phase diagram for carbon dioxide, you'll see it's always a gas under all these conditions -- it has to get really, really cold to make carbon dioxide ice, and you don't get that cold at the low elevations near Mars' north pole during the summer.
I took this phase diagram from an interesting article, "http://www.astrosociety.org/pubs/mercury/9801/snowball.html"
This may make you wonder, okay, if you read that diagram it looks like water should be stable as a solid under these conditions, so why did the chunks vaporize? The problem is that the diagrams assume equilibrium conditions. But Mars is a big system and there's heating during the day and cooling at night and dry or wet air masses moving in and wind doing stuff, so at any moment things aren't in equilibrium. I understand that there could be some conditions prevailing at the Phoenix landing site that might even lead to net deposition of water ice, but they've always seemed fairly sure that excavated chunks would sublimate during the day. (I wonder if any water ice deposition happens in cold traps overnight.) Large bodies of ice like what's exposed under the lander might show little change, sublimating a tiny bit during the day and getting deposited on a bit overnight. But if you sublimate much of a chunk during the day, it's gone for good; it's not like a chunk is going to reappear from nothing overnight, so chunks that are sufficiently small should go away as a result of cyclic sublimation and deposition.
--Emily
Posted by: deglr6328 Jun 20 2008, 06:03 AM
m! case closed. forgot about the relatively high temps right now at the landing site.
Posted by: alan Jun 20 2008, 06:16 AM
Water being stable as a solid at 8 millibars as indicated on a phase diagram would require a partial pressure of water vapor of 8 millibar not a total atmospheric pressure of 8 millibar. A layer of dirt on top of the ice could prevent the water vapor sublimated from the ice from diffusing away to the atmosphere so the vapor pressure of water vapor below the soil could remain near atmospheric pressure. Removing the dirt layer protecting it would allow the water vapor to disperse. Then the ice would then be exposed the the atmosphere which is mostly CO2 with little water vapor. Ice at the surface would be unstable and would sublimate unless the temperature was low enough to be at equilibrium with the partial pressure of water vapor in the atmosphere.
Posted by: silylene Jun 20 2008, 12:26 PM
Not to toss out the water too soon, but ....
There is also the possibility that this loss of bright stuff in the trenches could be water of hydration evaporating from a salt newly exposed to the open dry atmosphere. (water of hydration of a salt is NOT ice.) This would change the crystal morphology of the salt. This would then be expected to change the brightness/reflectivity of the salts. Frankly, I am surprised to see that everyone is proclaiming this ice without eliminating this possibility.
For example, magnesium sulfate exists as MgSO4.7H2O. The waters of hydration are lost in a very dry atmosphere over about 3-4 days. The hydrated form is bright and crystalline and reflective. The annhydrous form is dull and non-reflective. I remember doing this experiment as a freshman in college!
I want to see analytical proof of water. I really do hope this is water !
p.s. I found this very nice paper, discussion exactly these phase transitions in mag sulfate under martian temperature/pressure conditions, and expected physical appearance: CONVERSION OF CRYSTALLINE MgSO4.XH2O TO THE HYDRATED AMORPHOUS PHASE Ė A
RAMAN, NIR, AND XRD STUDY. http://www.lpi.usra.edu/meetings/lpsc2006/pdf/2168.pdf
Posted by: Juramike Jun 20 2008, 01:12 PM
Evidence of water ice now being reported on CNN:
http://www.cnn.com/2008/TECH/space/06/20/phoenix.mars.ap/index.html
(I have a minor issue with this sentence in the article: "However, an initial soil sample heated in a science instrument failed to yield evidence of water". - Couldn't they have said "did not determine evidence for water" instead of using the f-word?)
-Mike
Posted by: centsworth_II Jun 20 2008, 02:38 PM
Even if this could explain change in brightness of a surface, what about the entire disappearance of small, dislodged chunks?
Posted by: ElkGroveDan Jun 20 2008, 02:57 PM
It's probably time to start looking for evidence of rust on the footpads...
![]()
Posted by: ugordan Jun 20 2008, 03:00 PM
Yeah, Phoenix' price as used hardware might be going down. Nothing a good paint job can't fix, though!
Posted by: nprev Jun 20 2008, 04:30 PM
http://www.cnn.com/2008/TECH/space/06/20/phoenix.mars.ap/index.html
CNN TV also running the story, including a sidebar with Miles O'Brien that featured an animated before & after GIF very much like those seen here...is anybody at UMSF now a credited TV star, perhaps...?
Posted by: hortonheardawho Jun 20 2008, 04:43 PM
( briefly dropping out of lurking mode...)
As much as I hate to do so, I have to agree with silylene: the case for water-ice -- at least in the Goldilocks trench -- is "not proven".
http://www.flickr.com/photos/hortonheardawho/2595680622/ is a sol 20-24 3D animation of the shadowed area in the trench.
I created it to see if I could see how much of the clumps remained after the "sublimation". Unfortunately even the NASA gif image drops out in the darkest part of the shadow -- where the most interesting clumps were. But if you look closely at the bright clumps that fade nearest the shadow edges I think you can see that the clumps do not completely disappear.
Even in the darkest shadow there appears to be "something" where the white clumps were -- so, yes, there may have been a dramatic change in the white clumps -- but perhaps no more so than the reductiion in the brightness parts of the top of the trench.
Yeah, I believe that it's water-ice but it's not yet scientifically proven.
I am still puzzled why the infrared spectrum from the left SSI camera has not been cited as supporting evidence for water-ice.
( jumping back into cyber-space...)
Posted by: chris Jun 20 2008, 04:53 PM
Also unlurking briefly....
If the lumps were dirty ice, then surely you wouldn't expect them to completely disappear.
Chris
Posted by: Stu Jun 20 2008, 05:18 PM
http://www.nasa.gov/news/media/newsaudio/index.html on right now...
Posted by: mhoward Jun 20 2008, 05:37 PM
Big day for Mark and the rest of the team. Congratulations!
Posted by: ugordan Jun 20 2008, 06:04 PM
Indeed! It's awesome that an imaging instrument was able to beat other ones to the discovery and practically nail this as ice.
Posted by: brianc Jun 20 2008, 06:08 PM
ICE - wow that sure is COOL !
Posted by: CryptoEngineer Jun 20 2008, 07:58 PM
Very early after landing Phoenix took a look under the craft,
showing exposed bright material where the landing jets had blown
the sand away.
It would be interesting to revisit this area and see if there have
been any visible changes. This 'bright stuff' has been exposed
for much longer than any in the trenches.
ce
Posted by: marsbug Jun 20 2008, 09:14 PM
Yeah, I believe that it's water-ice but it's not yet scientifically proven.
I am still puzzled why the infrared spectrum from the left SSI camera has not been cited as supporting evidence for water-ice.
I agree that this doesn't prove the case for water, and that hydrated salts are a possibility, but I just don't see what you do on the animation: There are a lot of changes that could be due to different shadowing or image artefacts, but I don't see any consistent outlines between the two frames, and the shadowing around the lumps looks to change as well. Is there any chance you could draw me some outlines to make it a bit clearer?
Go on drop out of cyberspace, the view from here is just as interesting!
Edit; ICE, I meant doesn't prove the case for ICE, please oh great god of woo don't strike me down!
Posted by: bcory Jun 21 2008, 12:03 AM
Well the whole world knows now about the water ice
It's the main headline on the Drudge Report website ![]()
points to a Bloomberg article:
http://www.bloomberg.com/apps/news?pid=20601087&sid=aI10wpl35qqY&refer=worldwide
Posted by: CosmicRocker Jun 21 2008, 07:03 AM
Posted by: Oersted Jun 21 2008, 09:02 AM
Man, I really think this discovery, or rather confirmation, demands a new thread, something entitled "it IS ice"... Done - J
We now know for sure that Mars is full of rocket fuel and water to drink. Fantastic news.
Posted by: nprev Jun 21 2008, 02:08 PM
I think we've been pretty confident of that for several years now based on orbiter data, but it is indeed nice to see it at a "human" scale of reference...and at an easily accessible depth.
Now there's the problem of getting ice-laden samples into the ovens before it sublimates. CNN reported yesterday that a fresh dig had only about 30 min for delivery before ice crystals small enough to filter through the screens would sublimate off; is this accurate?
Posted by: ugordan Jun 21 2008, 02:27 PM
That's probably based on the telecon yesterday (Miles O'Brien was 'there'), when they said they wanted the whole process to take 30 minutes. That does not necessarily mean the ice would sublimate that fast.
Posted by: nprev Jun 21 2008, 02:39 PM
Thanks, Gordan! Had to miss the telecon; I'm in Texas attending a work-related class.
I would be curious to know just how rapidly suitably small ice crystals would sublimate during the daytime at the site, though. Presumably the team has a model for this; they'd need it to estimate the actual water content of the sample prior to acquisition.
Posted by: curious Jun 21 2008, 03:03 PM
showing exposed bright material where the landing jets had blown
the sand away.
It would be interesting to revisit this area and see if there have
been any visible changes. This 'bright stuff' has been exposed
for much longer than any in the trenches.
ce
Sorry for barging in, just a quick comment then I'll slink away to lurk-mode again.
These mission scientists are circumspect and they won't state things until they have absolute proof, and that's good. But:
As soon as we saw what got exposed by the rockets it was instantly obvious that anywhere they dug they would find more ice. By the flat heterogeneous landscape it's true for miles around. Phoenix is sitting on a frozen f'ing lake bed. Orbital data already says there is a vast amount of water ice underneath.
Other things that strike me as just 'obvious:'
They are digging these trenches side by side to make a clean workspace for going as deep as they can reach without surface material cascading into it, so as to get purer ice samples.
At this latitude the 'surface,' the depth to which sublimation happens at this time of year, is ideal. It's an awesome location for this experiment. They nailed it, absolutely perfectly.
But the real shock is yet to come, if it does: That will be if/when they detect amino acids in the ice makeup. On that discovery, they will be very, very circumspect - as they should be.
Very exciting times, it's truly historic. Water ice up close is unprecedented.
Thanks for this forum and to all who post, it's a terrific read. You do a superb job of getting down to details, which is why someone 'stating the obvious' is just a bandwidth-waster on one level, but is hopefully also perspective provoking and encouraging. Cheers
Posted by: ElkGroveDan Jun 21 2008, 03:07 PM
BTW have we had a look at Snow Queen lately? Ten bucks and my left (elbow) says it is going to sublimate away, albeit slower than the fresh samples.
The feature reminds me of those dirty ice chunks that build up under the wheel wells of a car and then drop on the street.
Posted by: nprev Jun 21 2008, 03:32 PM
Been wondering about that myself, actually; it's been awhile, but guess that the arm's been a bit busy. Seems like an important observation, though.
Re car wheelwell ice chunks, here's a quick story. When I first read about meteorites when I was little in the middle of a Montana winter there were apparently all kinds of things that looked like them on the road- black, sooty torn up 'rocks'. Spent an entire afternoon collecting & dragging these things into my backyard, convinced that I was doing important sample collection...my parents were a bit displeased later on after discovering a sizeable mound of crappy road ice back there!
Um, I was not a particularly bright kid...
Posted by: TheChemist Jun 21 2008, 03:51 PM
Aminoacids ??? I would not bet on it.
Phoenix is not equipped to detect organics, let alone find their structure.
The wet chemistry lab experiments will look for inorganics and oxidants, if I remember well.
Posted by: centsworth_II Jun 21 2008, 04:04 PM
This is one of the prime objectives of Phoenix. I don't know how specific they can get in the identities though.
If organics are found, chances are they would be the same types as already found in comets and meteorites... including amino acids.
"http://phoenix.lpl.arizona.edu/science_tega.php is sensitive to detection levels down to 10 parts per billion, a level that may detect minute quantities of organic molecules potentially existing in the ice and soil." (Let's hope they get those darn doors working!)
Posted by: elakdawalla Jun 21 2008, 04:25 PM
A number that's relevant to this discussion is what the maximum mass capability of the mass spec in TEGA is -- I can't seem to get that to turn up on a quick Google search. My guess is that amino acids are too large to be detectable -- that they're looking for smaller stuff, simpler carbon chains -- but that's just a guess, and it'd be helpful if someone could turn up a reference on this. IIRC, the mass spec instrument on Cassini can only detect the very smallest amino acid, whichever one that is.
--Emily
Posted by: nprev Jun 21 2008, 04:36 PM
Hmm. Thanks, Emily.
So, basically, the most wildly optimistic and hoped-for outcome would be to detect a plethora of simple products of thermal decomposition, which might hint at the existence of more complex molecules.
What are the design limitations of mass spectrometers with respect to identifying organics, anyhow? If I understand their operation correctly, they'll provide gross elemental composition with sort of a hint at relative abundances, but there's really no way to infer chemical structures except by comparison with standard "pure" spectra of a given substance.
Posted by: ElkGroveDan Jun 21 2008, 04:37 PM
IIRC glycine
(smallest that is, I have no knowledge of Cassini's abilities)
Posted by: belleraphon1 Jun 21 2008, 05:07 PM
Atomic mass limits for TEGA mass spectrometer:
"Phoenix Lander's Thermal Evolved Gas Analyzer: Differential Scanning Calorimeter and Mass Spectrometer Database Development
Author(s): Sutter, B.; Lauer, H. V.; Golden, D. C.; Ming, D. W.; Boynton, W. V.
Abstract: The Mars Scout Phoenix lander will land in the north polar region of Mars in May, 2008. One objective of the Phoenix lander is to search for evidence of past life in the form of molecular organics that may be preserved in the subsurface soil. The Thermal Evolved Gas Analyzer (TEGA) was developed to detect these organics by coupling a simultaneous differential thermal analyzer (SDTA) with a mass spectrometer. Martian soil will be heated to approx.1000 C and potential organic decomposition products such as CO2, CH4 etc. will be examined for with the MS. TEGA s SDTA will also assess the presence of endothermic and exothermic reactions that are characteristic of soil organics and minerals as the soil is heated. The MS in addition to detecting organic decompositon products, will also assess the levels of soil inorganic volatiles such as H2O, SO2, and CO2. Organic detection has a high priority for this mission; however, TEGA has the ability to provide valuable insight into the mineralogical composition of the soil. The overall goal of this work is to develop a TEGA database of minerals that will serve as a reference for the interpretation of Phoenix-TEGA. Previous databases for the ill-fated Mars Polar Lander (MPL)-TEGA instrument only went to 725 C. Furthermore, the MPL-TEGA could only detect CO2 and H2O while the Phoenix-TEGA MS can examine up to 144 atomic mass units. The higher temperature Phoenix-TEGA SDTA coupled with the more capable MS indicates that a higher temperature database is required for TEGA interpretation. The overall goal of this work is to develop a differential scanning calorimeter (DSC) database of minerals along with corresponding MS data of evolved gases that can used to interpret TEGA data during and after mission operations. While SDTA and DSC measurement techniques are slightly different (SDTA does not use a reference pan), the results are fundamentally similar and thus DSC is a useful technique in providing comparative data for the TEGA database. The objectives of this work is to conduct DSC and MS analysis up to 1000 C of select minerals that may be found in the martian soil. "
From NASA Technical Reports:
http://64.233.167.104/search?q=cache:QDVCYk7bkG8J:ntrs.nasa.gov/search.jsp%3FR%3D827370%26id%3D5%26qs%3DNe%253D25%2526Ns%253DArchiveName%25257C0%2526N%253D295%252B129+TEGA+MASS+SPECTROMETER+ATOMIC+WEIGHTS&hl=en&ct=clnk&cd=2&gl=us
Craig
Posted by: scalbers Jun 21 2008, 05:08 PM
--Emily
I saw a talk a couple of days ago by Emily Haynes, a Longmont area scientist/educator who will be spending some time in Tucson soon with the team. She showed an orbiter image with what to me looked like a small ice outcrop perhaps on the other side of Heimdall crater from Phoenix. So perhaps some ice can occur at the surface if it's in a shadowed area. The caveat I'll have to track down is whether this could possibly be dry ice instead. I'll have to look for this image as well.
Steve
Posted by: Juramike Jun 21 2008, 05:54 PM
Here is a link to a table of small organic molecules and their molecular weight (from Jovian/Titan-style atmosphere discharge experiment literature): (http://www.unmannedspaceflight.com/index.php?s=&showtopic=4003&view=findpost&p=91428)
With a detection limit of m.w. 144 (thanks, bellerapheron1!), the TEGA oven would be able to detect molecular weights that would get you up to aspartic acid (m.w. 133).
But remember, you'll only observe a peak at 133 amu. There are many combinations of atoms and structural configurations of those atoms that that could make the same peak. (As I learn everyday.)
(There are many ways to form amino acids, it doesn't necessarily infer life.)
-Mike
Posted by: curious Jun 21 2008, 06:49 PM
Microbes have survived on Earth for 90% of the planet's history despite all the climate change. Mars has had liquid oceans over long periods too.
They will easily be able to see bio-markers if they exist at this scale:
http://ares.jsc.nasa.gov/astrobiology/biomarkers/images.cfm
Long odds, but it's just possible these guys will be the first to see evidence of the Holy Grail they are after. And us too!
Posted by: scalbers Jun 21 2008, 06:52 PM
...and at meter scales could one look for such things like stromatolites?
Posted by: siravan Jun 21 2008, 07:14 PM
The discriminant power of a mass spectrometer depends very much to its resolution. IIRC, A good lab MS on earth has a mass resolution of 1 in 10^5. On the other hand, INMS on Cassini had difficulty telling N2 from CO in the Enceladus plume. The actual molecular mass of N2 is 28.014 and of CO 28.010 (a difference of 1 in 7000). In addition, each element has different isotopes and based on their abundance, the mass peak splits to multiple peaks with difference in mass of approximately 1. Glycine has a molecular mass of 75. But even if TEGA shows a peak at 75, it is difficult to claim it is glycine, unless it can resolve all the peaks and the relative ratio of the peaks follows what is expected from the isotopic abundance of C, O and N on Mars.
Posted by: TheChemist Jun 21 2008, 07:46 PM
If the soils have been heated up to 1000 oC, what is the chance that organic molecules with small enough MW will survive intact ?
Even if complex organics were there in the soil, it would be difficult to recognize their original structure by their low MW thermal degradation products.
And I concur that finding aminoacids in Mars would not be that ground shaking, these molecules are abundant ....
ok, back to watching Netherlands-Russia now :-)
Posted by: tedstryk Jun 21 2008, 07:51 PM
Yes, pretty much, save if something like this were to happen
Posted by: nprev Jun 21 2008, 07:56 PM
![]() ...good one, Ted, but unlikely. Phoenix is in the North polar area...
...good one, Ted, but unlikely. Phoenix is in the North polar area...
Posted by: tedstryk Jun 21 2008, 08:38 PM
True...I had a picture of a penguin with an almost mars-like background, which made merging the images easy enough to be worth the effort, but I did think of using a polar bear.
Posted by: Juramike Jun 23 2008, 06:39 PM
So if there is significant subsurface ice on Mars, does that mean that there could also much more subsurface granite than has been detected so far on the surface?
Here are the only references to martian granite I found:
THEMIS detects blob of granite on Mars: http://themis.asu.edu/discoveries-granitepeaks
Ruthorford and Hess, LPS 7 (1981) abstracts 915-917. "Granite genesis in a planetary context: processes and important variables for Mars." http://articles.adsabs.harvard.edu/cgi-bin/nph-iarticle_query?1981LPI....12..915R&defaultprint=YES&filetype=.pdf
-Mike
Posted by: ElkGroveDan Jun 23 2008, 07:08 PM
To be specific the THEMIS site notes "granite like" which includes an entire family of quartz-plagioclase-feldspar type rocks. They could very well be looking at diorite here, and as every good geologist will tell you......diorite should never be taken for granite.
Posted by: Juramike Jun 23 2008, 07:20 PM
I must bow to the Master.
Posted by: brellis Jun 23 2008, 08:55 PM
You guys rock!
Posted by: ElkGroveDan Jun 23 2008, 09:12 PM
That was gneiss of you.
Posted by: akuo Jun 23 2008, 09:54 PM
I looked at some of the older images. I think there is already evidence of ice subliming away in the images from sols 20 and 21. The colour combination in following images is from slinted:
The chunk on the left is in shadow in both images, and seems to be shrinking. Other smaller pieces seem to disappear altogether.
Posted by: nprev Jun 23 2008, 10:10 PM
Okay, you guys, cut that schist out!
Posted by: BrianL Jun 24 2008, 02:36 AM
I can only sit back and marble at the collective wit here.
Brian
Posted by: JRehling Jun 24 2008, 02:39 AM
My sediments exactly.
Posted by: Juramike Jun 24 2008, 03:04 AM
We have now Doug to a new level.
Posted by: Shaka Jun 24 2008, 03:07 AM
Geology's 100 Greatest Puns will return after these messages....
![]()
Posted by: dvandorn Jun 24 2008, 04:58 AM
I'll breccia anything we can't keep it going much longer, though... ![]()
-the other Doug
Posted by: CosmicRocker Jun 24 2008, 05:20 AM
Posted by: Shaka Jun 24 2008, 06:32 AM
Will someone explain to akuo what happened to his post.
Posted by: helvick Jun 24 2008, 07:20 AM
I don't know - I have a feeling this will turn into something of a clastic.
Posted by: imipak Jun 24 2008, 07:53 AM
This thread's going to be slated.
Posted by: PFK Jun 24 2008, 01:54 PM
They'll work themselves into a lava over it, no doubt. Mind you, some of the replies have been quite igneous.
Posted by: fredk Jun 24 2008, 03:21 PM
I predict that these replies will work their way further and further towards the porphyry of geological nomenclature, before spiralling down into a serious syncline.
Posted by: Ames Jun 24 2008, 03:27 PM
I'd love to ride across this land on a Horst and Graben a few rocks on the way.
sorry!
Posted by: cschmidt Jun 24 2008, 03:36 PM
Of quartz.
Posted by: nprev Jun 24 2008, 08:35 PM
I've never seen such twisted metamorphics in all my life. Is everybody on this thread...stoned?
(NOW I run like hell...)
Posted by: Rennmaus Jul 31 2008, 08:50 PM
Just confirmed: There is water on Mars: http://www.nasa.gov/mission_pages/phoenix/news/phoenix-20080731.html
Posted by: centsworth_II Jul 31 2008, 08:53 PM
This has to be the most confirmed fact about Mars EVER!
Posted by: bcory Jul 31 2008, 09:37 PM

I knew it when I saw the first pic of the"pond" ice was shown under the lander months ago 
" LOS ANGELES - The Phoenix spacecraft has tasted Martian water for the first time. The robot heated up ice in one of its instruments earlier this week. Scientists say the chemical test confirms the presence of ice near the Martian north pole.
Until now, the evidence for ice has been circumstantial. That was based on photos Phoenix took of a hard splotchy area near its landing site and changes it saw in a trench."
http://news.yahoo.com/s/ap/20080731/ap_on_sc/phoenix_mars_2
Posted by: Wildthing Aug 28 2008, 04:26 PM
(unnecessary chunk of previous post removed)
Loooong time lurker...first post...been wondering about this for a while....Has there been enough analysis done of the Martian "water" to make up a batch of it on Earth ??
Phoenix has "tasted" Martian water...what about us Earthlings ?? Is there eenough data on the water found on Mars to combine the appropriate elements (h2o +++ ??) and would it be safe for humans to actually sample ????
Posted by: Juramike Sep 3 2008, 10:59 PM
Phoenix has "tasted" Martian water...what about us Earthlings ?? Is there eenough data on the water found on Mars to combine the appropriate elements (h2o +++ ??) and would it be safe for humans to actually sample ????
Oh wow. Total marketing opportunity.
Once all the results are published, it would be pretty easy to figure out the right salts to mix up to come up with something that could approximate martian water as determined so far. (There might be trace elements present that were not detected or even some that could not be specifically identified).
You could probably leave out some of the elements and components to come up with something that might be non-toxic enough to sold. Slap a catchy label on it, a great ad campaign, and Martian Water could be all the rage.
-Mike
Posted by: stevesliva Sep 3 2008, 11:05 PM
Does Martian Water have antioxidants and lycopene?
Posted by: lyford Sep 4 2008, 12:14 AM
No, but it http://www.youtube.com/watch?v=jHElbD1imNo
Posted by: Juramike Sep 4 2008, 03:22 AM
So...first we sell the Martian Water....
......then we sell a water that has antioxidants to counteract any oxidizers in the Martian Water...
BWA-HA-HA!!!!
Posted by: dvandorn Sep 4 2008, 05:31 AM
Poison: $1
Antidote: $1 million
![]()
-the other Doug
Posted by: nprev Sep 4 2008, 07:51 AM
![]() ...best business model ever, you two!
...best business model ever, you two!
I suspect, though, that even faux Martian water would taste atrocious & smell worse, kinda like the tap water in Amarillo, Texas. If you leave a faucet dripping in that town for a week, you got yourself a sink stalagmite...
Powered by Invision Power Board (http://www.invisionboard.com)
© Invision Power Services (http://www.invisionpower.com)