Printable Version of Topic
Click here to view this topic in its original format
Unmanned Spaceflight.com _ Titan _ Atmospheric Chemistry of Titan
Posted by: Juramike May 2 2010, 03:38 AM
Here is a "Benzene-O-Vision" graphic showing the amount of benzene and phenyl radicals at high altitudes on Titan. This is based on detections of benzene and phenyl radical (which recombined in the sample chamber to make benzene) using the INMS instrument during closest approach. The numbers are normalized to constant pressure altitude, roughly 1000 km.
The data was taken from Table 1 in: Vuitton et al, Journal of Geophysical Research 113 (2008) E05007. "Formation and distribution of benzene on Titan". doi: 10.1029/2007JE002997 [EDIT 5/24/10: Article freely available http://www.lpl.arizona.edu/~yelle/eprints/Vuitton08a.pdf] and overlaid on a map of Titan.
The authors mentioned that the errors in these measurements are 20%.
These detections are well above the detached haze layer. Most are at the same sun azimuth angle. (T23 observation had the lowest angle.) Assuming that the temporal difference is minimal (each dot is from a different flyby), there doesn't appear to be an obvious correlation with latitude.
This graphic does show that benzene is present even waaaay up in the thermosphere and ionosphere.
Posted by: Juramike May 5 2010, 02:59 AM
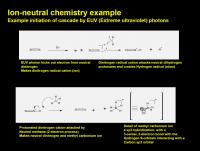
Into the Wierdness: Ion-neutral chemistry
(A major bummer about Titan chemistry literature is the lack of detailed electron-pushing mechanisms and especially frustrating is the lack of clear electron accounting. Many radical cations and even neutral radical in the figures do not show the unpaired electron on the structure. In my diagrams I'll try to detail the play-by-play.)
Much of Titan's chemistry is driven by upper atmosphere high-energy photochemistry. It is likely that plasma chemistry may play a small part, but the major driver is dayside solar radiation chemistry.
One of the biggest surprises of the Cassini mission is the amount of complex hydrocarbons (especially benzene!) found in the upper atmosphere. The thermo and ionosphere are pretty impressive chemical factories.
In these reaches ion-neutral chemistry plays a big role. The first step is the whacking of a molecule by a high energy photon (were talking Extreme ultraviolet, around 50-100 nm - this is mega-energy and waaay above your sunlamp). This is enough to blast an electron out of the molecular (or even atomic) orbital and create a wierd little species called a radical cation. This is a single electron process, so one of the electrons in the molecule is now unpaired, thus a radical. It is also charged positively, since an electron was ripped out of the system. Radical cations are pretty exotic here on Earth. They are usually only found in the vacuum ionization chamber of your local LCMS or GCMS. They do very weird things.
One of the things they like to do is bite into a covalent bond and make a charged species, and generate a neutral radical. (Think: Radical-cation + neutral --> Cation + uncharged radical)
Sometimes, structurally complex radical cations will frag up all by themselves in a similar way (radical-cation --> cation + uncharged radical). This happens in your local LCMS and GCMS, but that's a story for a different day.
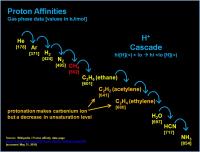
Here is an example that shows a nitrogen molecule (N2) getting whacked by a high energy photon, making a radical cation, then homolytically (single electron style) biting into a neutral hydrogen atom. One hydrogen gets stolen and gloms onto the dinitrogen to make a cation, while the remant hydrogen atom (now a radical since the electron is unpaired in the atomic orbital) goes flying off. But the dinitrogen cation is not happy, it has a very weak Proton Affinity and wants to give away the proton. When it finds a neutral methane molecule it can donate the proton to the methane molecule. (Y'all can think of it as an electophilic attack by the proton on the methyl, or a nucleophilic attack by electrons in the C-H orbital to the proton - either way it is the same). Compared to most stuff we are used to, neither methane or the nitrogen really want to be protonated. But in this case the nitrogen want the proton much less than the methane. So the methane molecule gets stuck with it for the moment. (Kinda like regifting an ugly sweater).
So you end up with a CH5+ cation. Which is weird. Drawing a pentavalent carbon is the best way to get ridiculed by your colleagues, or lose 10 points on a test score, depending on your situation. In the rarefied upper atmosphere of Titan, it is sorta tolerated (remember, the methyl isn't super happy about that extra proton). Structurally, two of the hydrogen atoms of the carbonium ion (normal R3C+ is a "carbenium" ion) are in a 3-center 2-electron bond. You can think of it as a hydrogen molecule sidebound to a carbenium ion. So these two hydrogens are in a special relationship. (But they can exchange out with the others in the structure).
Think of the methyl carbonium ion as a super acid. It wants to get rid of that proton (but can't to dinitrogen).
It turns out that CH5+ plays a special role, it actually prevents exciting chemistry from happening.
Posted by: ngunn May 6 2010, 11:20 AM
Thanks for that very interesting explanation Mike. As a non-chemist it's taken me a while to mull it over, alongside this related EGU abstract: http://meetingorganizer.copernicus.org/EGU2010/EGU2010-10730.pdf
I am intrigued by the possible implications of this for the chemical erosion of Titan's solid surface materials. Unfortunately the abstract, not being one of those long LPSC ones which spoil us rotten, doesn't include the actual findings on how the 'bedrock' (whether H2O, H2O/NH3 or solid organics) are or might be affected. Do you have any more info/ideas on this? Could this carbonium be what eats up any exposed ammonia on very short timescales?
Posted by: Juramike May 6 2010, 01:50 PM
The radical cationic, carbonium, and carbenium species up in the atmosphere are high energy intermediates. They can exist up there (and in the MS sample chambers) because they are in rarefied environments. They don't bump into other molecules that often.
As these compounds descend in the atmosphere, they will encounter other molecules, bump into things more often, and be able to exchange energies and react much, much better. When you get down to the surface (or in solution) there are a lot of opportunities for molecules to react and find a happy equilibrium. High-energy structures will be just fleeting intermediates on the way to more more stable molecule. They won't be able to last more than a molecular vibration before they bump into something and react or transfer their energy.
'Course at Titan's low temperatures, some of the metastable high-energy compounds might get trapped out in a matrix and not be able to react. One of the best ways to keep things from reacting is to cool it down, putting it in the freezer so to speak and keep it from getting over the energy hump to the next state. If there is a high energy transition state with higher activation energies away from it, you can freeze it out. (IIRC, carbenes [think of it as "methylene diradical" or :CH2] has been studied in a frozen Argon matrix).
I'm not real sure about the Lunine abstract....methane is very non-polar and would not be happy solvating a carbocation. I think it would be EXTREMELY difficult for methane to spontaneously dissociate to a carbocation-hydride. (This doesn't happen in the lab at terrestrial temperatures.) But if a carbocation (or superacid) was plopped or sprinkled into a lake, it would do something.
Definitely not comfortable with referring to normal methane (CH4) as a "protic" solvent. That implies that methane is able to donate a proton. (CH4--> -:CH3 + H+). With a pKa of 45, that won't happen.
BUT (and I think this is where the abstract is going) if a stronger superacid was sprinkled into a lake, the proton affinities might force methane to take on a proton and make CH5+ (again, methane is not happy about this.) Proton affinities show the clear progression CH4-->CH3-CH3->acetylene-->ethylene-->H2O--<ammonia. (Table here: http://en.wikipedia.org/wiki/Proton_affinity_%28data_page%29). So if CH5 (or H3+) was dribbled into a lake it would eventually work itself down the chain to protonating water to make hydronium. (Dunno the proton affinity for clathrate, but I assume it's similar or slightly less than water, protonation should break up the clatrate matrix - that would be a neat and "easy" experiment to try.) The ultimate sink on Titan should be ammonia to generate ammonium ion.
Posted by: Littlebit May 11 2010, 09:22 PM
Is methane+ a right wing or left wing radical?
Enjoy your posts, as always Juramike - we are still out here!
Posted by: ngunn May 11 2010, 09:56 PM
Could be either way, depending on the geometric orientation of the molecule and where you view it from.
Posted by: Juramike May 12 2010, 12:30 AM
Cute.
To be really, really anal, a radical should show a dot to signify an unpaired electron. So .CH3 or (neutral radical) or .+CH3 for the radical cation.
Differently trisubstituted carbon radicals .CR1R2R3 could be in sp3 orbitals. So technically, they could be chiral (have handedness / are not superimposable on their mirror-image) if the three groups are different. The resulting radicals could be chiral (all sp3 hybridization, or may be achiral and planar (unpaired in p, bonding in sp2 orbitals) and react differentially from the two faces to create chiral products.
A trisubstituted carbenium ion +CR3 will rehybridize to placing the unoccupied orbital in a p atomic orbital, and the remaining (bonding) orbitals in sp2 orbitals. This is a planar configuration, and is thus achiral.
As Professor Barry Sharpless once said while teaching us organic chemistry: "Once you lose your chirality, it is gone forever." ![]()
Here is a neat basic intro to radical chemistry: http://www2.fiu.edu/~herriott/ch10%20-%20radical%20reactions.pdf
Posted by: Juramike May 12 2010, 11:46 PM
Purple Haze or Where It’s At
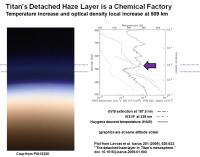
A paper by Lavvas et al. (Lavvas et al. Icarus 201 (2009), 626-633. "The detached haze layer in Titan's mesosphere." doi: 10.1016/j.icarus.2009.01.004) examined the detached haze layer and reported that it is the major driver of Titan chemistry.
The graphic below shows that the detached haze layer (the image and plot are scaled to the same altitude) has a shift in the UV-Visible properties (187 and 338 nm) at about 520 km above Titan. This also corresponds to an inversion layer measured by the Huygens probe (solid line in plot). So what makes up this warm thicker layer?
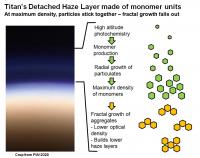
The authors state that photochemical production occurs very high up in Titan’s atmosphere, near 1000 km. Some of these chemical reactions produce particles which slowly grow radially as they thicken downwards. At about 520 km, these monomeric particles reach their highest optical density forming the visible purple layer, and absorbing solar radiation and causing the local warm region in the upper atmosphere. But at this high density, the particles begin to glom together and stick to form even larger aggregates. Fractal growth begins, and the agglomerated particles fall through the upper atmosphere forming the larger globby particles that make up the lower haze layers. According to the authors, the agglomerated particles have a lower optical density than the individual monomers (many small particles = hi optical density; one biiig particle = lower optical density), so the region immediately below the detached haze layer is “clearer”.
The authors created a model with a photochemical production centered around 900 km altitude and a mass flux of 9E-14 g cm-2 s-1 and an average particle size at 520 km of 40 nm which fits the observations and fit the required influx rate for the Titan’s lower stratospheric haze layers from above.
It is very important to note that the saturation vapor pressure of simple organics (such as benzene) is not high enough to condense out at 500 km. The aerosol particles are made of bigger molecules.
The chemistry that occurs at these high altitudes is driven by solar flux of high-energy electron-stealing EUV photons around 145 nm. The solar flux also matches the modeled production rate.
Here are some numbers:
Overall photochemical mass flux (organics+aerosol particles): 9E-14 g cm-2 s-1
Aerosol production flux at 520 km: 2.7-4.6E-14 g cm-2 s-1 (30-50% aerosol particulate production)
Particulated Flux required to maintain stratospheric haze layer: 0.5-2.0 E-14 g cm-2 s-1.
The high-energy photochemical pathways up at 900 km drive much of Titan’s organic chemistry, making organics (such as benzene, seen at 1000 km) and particulates.
Posted by: Juramike May 16 2010, 09:52 PM
Titan's organic chemistry is driven by photochemical reactions that originate high in the atmosphere, near 1000 km, where the pressure is only 1.5E-5 Pa. (This is 1E-10 atmosphere, or 1/10 billionth Earth's pressure, much much weaker pressure than I got in my graduate school "vaccum" line. This is about the same range as a typical high-vacuum molecular turbopump.)
Taking 1.5E-5 Pa altitude as an upper limit for stuff to happen, here is a graphic that shows the "chemically available" atmospheric volume for each of the planets:
(full res here: http://www.flickr.com/photos/31678681@N07/4612160825/)
As expected, Jupiter dominates, but Saturn may actually be more impressive, with a lower gravity, the scale height is larger - it has a fluffier atmosphere. For similar reasons, smaller Neptune has more atmosphere. Values for Saturn, Uranus, and Neptune are based on scale height - which is probably underestimating the 1.5E-5 limit. The Jovian estimate is based on Galileo probe data.
Same graphic but detail for the silicate and icy bodies:
(full res here: http://www.flickr.com/photos/31678681@N07/4613181600/)
Titan has more atmosphere, and it extends farther out, so it's chemically-available volume is larger than Earth's. With more mass and higher density, both Earth and Venus hold their atmosphere in closer. With a low surface pressure, but a much weaker gravity field, Pluto has a large but thin atmospheric volume.
Ganymede, Callisto, the Moon, and Mercury have a surface atmosphere well below the 1.5E-5 Pa cutoff and so are not shown.
The atmosphere chemistry that happens on Titan depends on amount of atmosphere, amount of solar flux, and the exact mix of the methane-containing atmosphere (spoiler alert: H2 is bad, N2 and Ar are good).
For other methane-containing atmospheres, it might be possible to scale Titan's atmospheric chemistry to derive photochemically derived organic fluxes on those surfaces as well. (Hint: Pluto!)
Posted by: Juramike May 18 2010, 12:30 AM
Titan's Chaotic Chemistry
Titan is a synthetic chemist's worst nightmare. There are high energy processes that span the spectrum (!) of energies and modes of reactivities, plus the products of one reaction become the reactants for another. One recent model takes into account over 415 simultaneous reactions in the atmosphere. Even with all this complexity (a polite word for chaos), some of the models are beginning to get close to the observed Cassini and Huygens results.
One of the tricky bits is that Titan has different levels of reactivity. High-energy photochemistry (ion-neutral) chemistry dominates the upper atmosphere, while lower down "lower energy" radical reactions come into play. Finally, even pi-system photochemistry kicks in with longer wavelength light (200 nm or so) that is able to push with double bonds and pi-systems into an excited state. Once in the excited state, all sorts of things can happen. Photochemistry with excited states can occur even near 0 K. Deep below the haze layers, all the fun photons are absorbed, and the chemistries rely on ground state thermal chemistry - the day to day stuff we are used to. Here the energy barriers need to get crossed by the kinetic energy of the molecules themselves. And on frigid Titan, the molecules are gonna struggle to get up over that hill and over into the next valley. Here is a graphic that tries to put that all into perspective, with an example transformation for each type of reactivity:
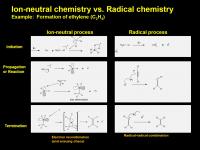
The next graphic compares an ion-neutral route and a radical route to ethylene starting from methane. Both occur in Titan's atmosphere:
Of the two types of chemistry, ion chemistry is the more energetic, during the initiation it rips an electron out of the molecular or even atomic orbital. The radical cation either reacts or self-fragments to generate a cation AND a radical species. (two reactive intermediates!). Further reactions proceed until the last step where an electron eventually collides with the system. It took a lot of energy to rip the electon out, and when the electron is popped back in a huge amount of energy is released. This huge amount can't easily be released just through wimpy vibrational or translation mode changes - instead the molecule may frag up. (Picture driving your car along the road, suddenly two solid rocket boosters you've been carrying along are ignited, and your cars structural frame can't absorb the extra energy....) So even at the end, ion neutral chemistry will generate reactive radical intermediates that enter a radical reaction pathway...
In the scheme, a methyl group loses an electron, then blows out a hydrogen radical. The resulting cation sucks in the electrons from a methane molecule (I drew it as a 2e- exchange, it could be several 1e- exchanges also). This leaves it as a C2H5+ cation and kicks out molecular hydrogen (again, it could kick out two H radicals). [C2H5+ is a real important intermediate in Titan chemistry, we'll see him again, soon.] At some point, an electron drops into the system, with a huge release in energy. The least exciting option is a fragmentation to ethylene and the release of yet another hydrogen radical.
For the radical route, a homolytic cleavage (1e- split) creates a methyl radical (CH3.) and a hydrogen radical (H.). Both the methyl radical and hydrogen radical can go on to react with other species (H abstraction or addition to a double bond, for example.) In this scheme, the Hydrogen radical reacts with acetylene to create an ethylene radical, which can then propagate to do further stuff. To cut this story short, the ethylene radical encounters a hydrogen radical, they combine and make a neutral molecule full of happy paired electrons, ethylene. (In reality, it is an encounter with another ethylene radical that causes a disproportionation to ethylene and acetylene, the more energetic ethylene radical likely loses a hydrogen radical to another ethylene and is converted to acetylene.). Once the electrons are paired up, that terminates the propagation.
The two types of chemistry are very important in planetary atmospheres. It was the realization and inclusion of ion-neutral chemistries at the proper level of importance that has generated the most recent round of models. And these were driven by the discovery of large amounts of benzene in Titan's atmosphere (see post #1).
Posted by: Juramike May 20 2010, 04:17 AM
Many ways to fall
Below is a graphic showing an energy diagram for two photochemical methyl neutral cleavage pathways:
A typical energy diagram starts at one energy level, passes through a highest level transition state (the high energy point - indicated by bracket "TS"), then might drop down to an local minimum intermediate state, then climb back up to another transition state before dropping down to a lower energy state for the end product.
Going from the midpoint to the right, a methyl-hydrogen bond cleaves homolytically (one electron goes each way) to form two radical intermediates. This can react with another methyl radical (slight energy increase due to the initial electron repulsion of the orbitals - gotta have enough speed to overcome that) to then join into a formal bonding arrangement and make ethane. In this case the two unpaired electrons jump in together to form a new bond.
To the left, there is another option. Two electrons from the methyl C-H bond can jump over to a neighboring hydrogen to make an H-H bond. Simultaneously, the two electrons in the second C-H bond can jump onto the carbon atom. In this case both electrons move as a pair, and there are two pairs moving simultaneously in a four electron process. The two electrons on the carbon are paired up and this species is referred to as a singlet carbene.
Singlet carbenes have a new reaction pathway to them, they can muscle in and insert directly into a C-H bond. This is a mode of reactivity displayed by many metal species - in fact, C-H activation/insertion has been one of the really hot topics in organic and organometallic chemistry in the last 20 years. I always think of it as a transient 3 center 4 electron bond just before the molecular orbitals switch to the incoming carbon center. This allows a singlet carbene to insert directly into a neutral unactivated methane molecule.
EUV photons have a heckuva lot of energy. A 100 nm photon can deliver a whopping 288 kcal/mol. This is enough to break any bond in the system and overcome a lot of activation energy barriers including either the radical or singlet carbene pathways above.
And it gets uglier.
There are even more possibilities for the neutral methane dissociation, shown below:
Aside from the diradical and singlet carbene pathways, there is also a triplet carbene pathway (think of as a diradical, both electrons are unpaired in different orbitals.) which spits out two hydrogen radicals. There is also the possibility of spitting out three hydrogen atoms one as H2 and one H radical. (thus leaving the electron deficient C-H with two paired electrons in one orbital and one unpaired.) With a Lyman alpha photon (flux responsible for 75 of the neutral methyl cleavage) the different pathways go in different amounts, with the radical pathway #1, and the singlet carbene as #2.
[There is one pathway that doesn't go well at this wavelength, that is the reaction where two hydrogen radicals leave but the carbene is in a singlet state, this shows at some point the triplet carbene went into a singlet state, a classic example of an intersystem crossing, which is technically considered a "forbidden" transition. Not to say it can't ever happen, it's just not cool from a symmetry point of view and is thus disfavored.]
Not mentioned here, but still possible at higher energies, is the "total fraggo" option where only a carbon atom remains with four electrons.
Thus, the high-energy inititiation of a neutral cascade by photodissociation has many different pathways. Similarly, high-energy ion-neutral cascades also have many possible pathways for each intermediate.
In the next post, we'll see how the initial ion-neutral photodissociation of methane is affected by the other atmospheric components, whether nitrogen, argon (both activators for aromatic formation) or hydrogen (inhibitor of aromatic formation.) This represents the fork in the road between Titan atmospheric chemistry (lotsa aromatics), and Jovian/Saturn atmospheric chemistry (not-so-much aromatics).
Posted by: rlorenz May 20 2010, 07:17 PM
A paper by Lavvas et al. (Lavvas et al. Icarus 201 (2009), 626-633. "The detached haze layer in Titan's mesosphere." doi: 10.1016/j.icarus.2009.01.004) examined the detached haze layer and reported that it is the major driver of Titan chemistry.
A major thrust of the paper is that the detached haze layer is located by the chemical fluxes alone (i.e. they dismiss the
previously-proposed dynamical origin)
I disagree. The detached haze varies both with season and latitude, so I don' t think things are as simple as
their 1-D perspective claims.
Posted by: Juramike May 21 2010, 05:42 PM
I agree that the reality is probably a lot more complicated than the model, with changing solar flux rates (11-year cycle) and seasonal illumination changes, and material (starting materials and products) fluxes and thus concentration values going up and down and then the molecules themselves physically going up and down and back and forth. All of those should also affect the chemical flux rates.
IIRC the mean free path length at 1000 km altitude is a few km long, so only a few ricochets are in the way of a 100 km molecular journey.
(I seem to also remember that Voyager 1 had a different value for the detached haze layer, much lower if I remember correctly.)
What measurements would be required to constrain chemical flux vs. dynamics?
(...and did we just get these on the last flyby?)
Posted by: Juramike May 31 2010, 04:57 AM
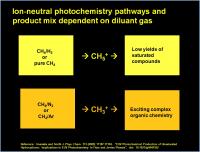
It turns out that the methane is only part of the story. The other non-carbon containing gases present also make a huge difference in the types of chemistry and the amount of products. An interesting experiment was done by Imanaka an Smith (Imanaka and Smith, J. Physical Chem. A. 113 (2009) 11187-11194) as summarized in the graphic below:
The authors found that when methane was irradiated in the presence of Argon or nitrogen, they got lots of unsaturated complex organics. In pure methane, the yield dropped, which is counterintuitive, since there is more methane in pure methane. And when methane was diluted with hydrogen, the yield dropped and only simple saturated compounds were produced. The best yeilds in their study was when methane was diluted with N2 or Ar. This is strange, because N2 or Ar are not even incorporated in the products, what gives?
A detailed look at the chemical mechanisms involved reveals the answer…
Posted by: Juramike Jun 1 2010, 03:36 AM
In the presence of N2 or Ar, ionization occurs first in the carrier gas to form either N2 radical cation or Ar radical cation. Abstraction of an electron from methane (CH4) also splits out a hydrogen radical, generating CH3+ a carbenium ion.
In the presence of H2, the photoelectron ejection creates a molecular hydrogen radical cation (= two protons with only one lonely electron in the molecular orbital keeping it all together..). This reacts with another H2 molecule to grab a hydrogen atom and also split out a hydrogen radical. This forms H3+, which is another 3-center, 2-electron triangular association. This is a very common molecule in deep space. But it is not happy. It has a very low proton affinity. It wants to give the proton away. (Fun fact: only dioxygen, argon, neon, and helium have a lower proton affinity – that means that H3+ can protonate (transfer it’s proton) to everything else – it is almost the ultimate superacid.) It is so strong a superacid, that it can actually protonate methane to make CH5+, a carbonium ion. This also has a 3-center, 2-electron triangular association. This time it is between the sp3 hybridized orbital on the carbon, and the two 1s orbitals of the hydrogen. The two electrons are shared among these three orbitals.
In the presence of pure CH4 (graphic above), electron ejection generates methane radical cation, which steals a hydrogen atom from methane to create CH5+ and a remnant CH3 radical. As we will see much later, the CH3 radical participates in radical chemistry processes that lead primarily to simple saturated organics. To get the really good funky stuff, we need to make ethylene or other unsaturated intermediates.
So N2 and Ar atmospheres (with CH4) generate CH3+, but CH4 and H2 atmospheres generate CH5+. That is the key difference. The downstream chemistry of these two cation intermediates determine the product mix.
Here's my memory trick:
Carbenium ions (CH3+) are fun.
Carbonium ions (CH5+) are boring.
Posted by: Littlebit Jun 1 2010, 07:59 PM
Carbenium ions (CH3+) are fun.
Carbonium ions (CH5+) are boring.
So three's a threesome and five is a crowd?
Fun stuff Mike. Ok, fun to a few of us. It seems like, with all the radical chemistry we should find more nitrated compounds. Where are they and/or why not?
Posted by: Juramike Jun 1 2010, 08:34 PM
Nitrogen does get incorporated. It is primarily in the form of nitriles (RCN). There should be surface deposits of cyanide (HCN), acetonitrile (CH3CN), and acrylonitrile (H2C=CHCN), cyanoacetylene (HCCCN), and cyanogen (NCCN).
Tholins are also thought to be rich in nitrile functionalities.
I'm still digging through some of the literature, but it seems that the literature rates for nitrile formation are a little less nailed down than for hydrocarbon formation processes. The key starting point seems to be molecular dinitrogen getting blown apart to form nitrogen radical carbene (.:N:).
Posted by: Juramike Jun 2 2010, 04:15 AM
So what does CH5+ do? Basically(!), not much. It is just a transporter for a proton that it really doesn’t want. Once it finds a better proton acceptor (HCN used as an example in the graphic below), it gives the proton away and reverts back to a happy neutral CH4 molecule. All done.
CH3+ on the other hand, is an electron-deficient intermediate. It wants electrons - badly. It is willing to steal them from another molecule. For instance, in the graphic above, CH3+ steals electrons from a methane (CH4) molecule, kicking out a hydrogen molecule in the process. These are all 2-electron processes and are shown with a full double-headed arrow. AS the two electrons from a C-H bond goes to form a new C-C bond, another two electrons from another C-H bond jump to make a bond between the two H atoms, thus making a dihydrogen molecule.
The end result is an ethyl cation. This is more stable since the electron-deficient carbon’s empty orbital has partial overlap with one of the C-H orbitals. This is called hyperconjugation. Carbenium ion stability is tertiary>secondary>primar>>methyl.
The ethyl cation can be thought of as an electron-deficient ethane cation. But it can also be thought of as protonated ethylene. If a suitable base is around, it can pass off the proton (methane wants it even less, but HCN will accept it) and generate ethylene. Ethylene is unsaturated and has a lot of new modes of reactivity due to the double bond – we’ll be seeing it much more. One can build a lot of neat things up from ethylene, and thus CH3+ is the grandaddy of all the neat stuff.
The game of proton “hot potato” cascades down due to the energies of proton affinity. Protons really, really hate to be alone. Even protonated helium is better than a helium atom + a lonely proton. The following graphic shows a big proton cascade for many Titan-relevant molecules:
It starts up high then goes down to the left. This chart and energy numbers are only relevant for gas phase species. Energies in the liquid (or even solid) phase will be different due to better solvation of the proton and protonated species (which is also solvent dependent – protonated water in hydrocarbon solvent will be less happy than if it was protonated water in water). Still, it should be no surprise that ammonia or other organic amines should be the ultimate proton sink on Titan’s surface.
Posted by: Juramike Jun 3 2010, 03:32 AM
Even on electron quenching of the CH5+ radical, it is pretty unimpressive. It pretty much goes to CH3 radical and 2 H radical. Although these are mildy exciting, it is nothing compared to what can happen to CH3+.
On e-quench of CH3+, the amount of energy released blows the system apart to generate an even more reactive carbene some of the time. The rest of the time, it goes to even more reactive intermediates, the radical carbene .:CH (whoa!) or even the total-fraggo uber-reactive option to a carbon atom.
So even if these intermediates manage to not react with other species and finally hook back up with an electron, CH3+ generates the more reactive and exciting intermediates.
Posted by: ngunn Jun 3 2010, 09:01 PM
About Titan chemistry, not necessarily atmospheric:
http://saturn.jpl.nasa.gov/news/newsreleases/newsrelease20100603/
Posted by: Juramike Jun 4 2010, 12:38 AM
Great catch!!
From the press release: "We have a lot of work to do to rule out possible non-biological explanations. It is more likely that a chemical process, without biology, can explain these results – for example, reactions involving mineral catalysts."
For a quick peek at what kind of reactions acetylene can undergo, see some of the discussion in this [lengthy] post: (http://www.unmannedspaceflight.com/index.php?s=&showtopic=4003&view=findpost&p=127195)
Posted by: Bill Harris Jun 4 2010, 10:02 PM
Mike,
This has been a good discussion of the chemistry of Titan's atmosphere. A lot of food for thought.
I'm not a chemist (except for a smattering of geo-chem), but this is my view of Titanian (??) atmospheric chemistry:
The atmosphere is at a higher pressure, and since the gravity of Titan is less, this density varies less with altitude.
The atmospheric temperature of -95*K or so also gives an effectively dense atmosphere with small distances between molecules.
At this low temperature chemcal reactions take a very long time to occur and even intermediate compounds that are unstable under Earthly conditions are stable for subsequent reactions on Titan. We've probably not even thought of the catalysts that can be created.
With no magnetic field of it's own, Titan's atmosphere bears the full brunt of the solar wind, as well as cosmic radiation which add enough energy with that ionizing radiation to initiate some reactions.
Titan's atmosphere is a reaction vessel of, uh titanic proportions, under unearthly conditions.
And at the surface of Titan, with water/ice and methane, nitrogen and ammonia there are undoubtedly clathrates/hydrates under unearthly conditions.
--Bill
Posted by: Juramike Jun 4 2010, 10:49 PM
The low temperatures at the surface and lower atmosphere (<100 km) will hinder most thermal reactions we would consider easy at terrestrial temperatures.
But up high, the high energy photons + the thicker atmosphere at high altitude with methane make for a lot of really high-energy exotic chemistry. The really fun stuff happens above 200 km altitude. There's a peak at about 300-400 km, and another peak about 800 km, as different processes have their preferred altitudes.
So the fun stuff happens up high, then the products condense out around 130 km, then stays inert down to the surface.
[Some of the fun intermediates could get trapped and embedded in haze particles that make it down to the surface. (I'm guessin' that is the crux of the Lunine et al. abstract). Even at low temperatures, a carbenium ion thrown in solution with some unsaturated compounds might do some reacting.]
Aside from all this, one really important point is that photochemistry can occur near 0 K. So while ground-state (thermal) chemistry might be difficult at low temperatures, once you've photoexcited something, even at very low temperatures, you can do exciting (!) things. [Earths ozone layer protects us from these photons by absorbing the light and cycling through oxygen species.] So even in the cold haze decks of Titan, as long as UV can penetrate, it will do stuff.
Posted by: Juramike Jun 4 2010, 10:52 PM
For the moment, the Clark et al. paper can be found here by scrolling through articles in press: http://www.agu.org/contents/journals/ViewPapersInPress.do?journalCode=JE
Unfortunately, access is required for any detailed information. There is not yet an abstract.
Posted by: Juramike Jun 6 2010, 07:58 PM
Article in press: Strobel, D.F. Icarus (2010) Article-in-press. "Molecular hydrogen in Titan's atmosphere: Implications of the measured tropospheric and thermospheric mole fractions." doi: 10.1016/j.icarus.2010.03.003
If I understood this correctly, the models for H2 flux don't match up to the observations. This paper attempts to "follow the hydrogen" and do some atomic accounting to see how well the hydrogen matches up to models.
H2 is made by a lot of the atmospheric chemistry processes. (All those H. radicals and H2 on the back side of the equations.)
Much of it escapes from Titan off the top of the atmosphere. But according to the author, an equivalent amount goes down and away onto Titan's surface.
(6.6E27 H2 s-1 escape vs. 2.9E27 H2 s-1 down to surface and away : I'm assuming the units are actual molecules - thus 10600 moles of H2 escapes Titan every second.)
One interesting possibility the authors raise is that the H2 is sucked up into surface (or subsurface) reactions with heavier organics that then regenerate ("crack") CH4.
Even with this, the overall atmospheric escape of H2 still ensures that methane will eventually be converted to heavier organics.
Key quote: "...either the observations are not consistent with each other or that our understanding of CH4 and H2 photochemistry is flawed and needs some revision."
Since a lot of the molecular production rates for other species are bouncing around by orders of magnitude (I'll post a table soon) from one model to the next, it is not surprising that the hydrogen production rate is also not-so-well constrained. Titan chemistry is complicated.
Posted by: Juramike Jun 8 2010, 12:43 AM
H2 is the key reagent that shunts the pathway to the CH5+ intermediate. Atmospheres with large amounts of H2 will generate CH5+ and thus give low yields of saturated organics, while planetary atmospheres with N2 as the diluant will have a richer organic chemistry via the CH3+ intermediate.
CH5+ (H2 present, boring chemistry): Jupiter, Saturn, Uranus, Neptune
CH3+ (N2 diluant – little H2, exciting chemistry): Titan, Pluto, Triton
Working out the full atmospheric chemistry of Titan will also help understand and be a possible preview for some of the chemistry that could occur on Triton and Pluto. It also provides a more complicated example of the carbon-based chemistry that could be happening on Jupiter and the other gas giants. It will also help shed light on processes that can occur on moons and planets and moons outside our solar system that have methane, and atmospheres with either H2 or N2/Ar.
Posted by: Juramike Jun 9 2010, 02:16 AM
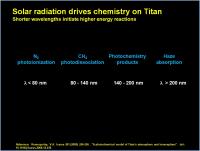
Titan’s atmospheric chemistry is driven by sunlight, and the fact that CH4 is present with large amounts of N2 as the diluant. The different wavelengths of light will hit different types of bonds, and will also penetrate to different depths. Very short wavelength photons (EUV) will not penetrate very far before hitting a nitrogen molecule and effectively blowing it into high-energy pieces. Slightly longer wavelength photons will penetrate a little deeper, only because the molecular transitions that would absorb this energy, located on methane molecules, are few and far between, the atmosphere is still composed of nitrogen molecules (95%). The “longer” UV wavelengths, powerful enough to excite double and triple bonds, penetrate deeper still (they are too wussy to interact with N2 or CH4 – they just pass on by) until they can find ethylene, acetylene and similar compounds. Finally, the longer wavelength UV light, the kind that gets absorbed by extended conjugated or aromatic pi-systems, penetrates to the haze layer. It also gets scattered by the haze particles.
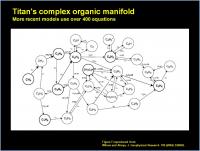
Once initiated, Titan has a complex reaction manifold that simultaneously creates and uses intermediates to make organic products. Here is an oversimplified diagram that shows some of the intermediates and routes:
Each intermediate has several simultaneous routes for formation as well as for reaction (destruction) to make other products. Each reaction pathway has its own rate. Recent models have over 400 simultaneous rates. It is sum of the the simultaneous inbound, and simultaneous outbound routes that determine the overall amount (flux rate) of a given intermediate that gets made. And yes, this varies by altitude (and temperature) as well. So the 400+ simultaneous rates at 100 km will be different than the 400+ simultaneous rates at 1000 km.
Here is a table that summarizes the overall surface flux rates for many of the predicted common organic compounds on Titan:
It would only take one little change in one of the rates to propagate through the system and change all the values. Thus, it is not surprising that each author’s total flux rates are different.
For example, note that some authors predict huge amounts of ethylene being formed, while others predict none at all. Where none is predicted, it is because there are other processes that consume ethylene as fast as it is produced. Obviously, a measurement of C2H4 (m.w. 26) from the burp the Huygens GCMS measured on the surface would help constrain these models and kick off a whole new round of model improvements.
Posted by: remcook Jun 9 2010, 07:04 AM
Starting on Monday: http://fd147.univ-rennes1.fr/articles.php?lng=en&pg=10 , which is heavy on Titan chemistry.
Posted by: Juramike Jun 10 2010, 02:58 AM
Very cool!
Here is an EXCEL conditional formatted graphic that shows how the predicted fluxes have varied between one published model and the next. These are all surface height ratios (m.w. and predicted density taken into account) normalized to values from the Krasnopolsky 2009 model. Yellow-orange means it is ballpark same order of magnitude. Dark red shows that the model underpredicts relative to Kransopolsky 2009 by over an order of magnitude, while dark blue shows that the past model overpredicts the Krasnopolsky 2009 by over an order of magnitude.
As yet another example, 1-butene (CH2=CHCH2CH3, m.w. 56, a liquid at Titan temperatures) has some of the greatest variation between all the models.
Posted by: Bill Harris Jun 10 2010, 02:31 PM
This is mindboggling. The potential scope of the chemistry on Titan is almost beyond comprehension.
Life is simply organic chemistry in action, with C-H-O-N reacting in a consistent and useful way. I don't want to get too philosophical about this, we may be the result of carbon being pre-programmed to act as carbon does.
Wonderfully informative charts and diagrams-- thanks, Mike.
--Bill
Posted by: Shaka Jun 10 2010, 11:59 PM
Will the final exam be essay or multiple choice? ![]()
Posted by: Juramike Jun 11 2010, 12:30 AM
I'm gonna give away the answers! ![]()
Posted by: Juramike Jun 11 2010, 05:51 AM
Enter the mechanism
Over the next several graphics, I will detail the formation mechanism for many of the predicted Titan organic species. I will show the dominant pathway based on rate information found in Krasnopolsy, V.A. Icarus 201 (2009) 226-256. "A photochemical model of Titan's atmosphere and ionsosphere". doi: 10.1016/j/icarus.2008.12.038. Happily, this article is publicly available and can be downloaded freely here:
http://pagesperso.lcp.u-psud.fr/pernot/ISSI_1D/Biblio/Krasnopolsky09.pdf
I will be referencing the reaction Id number in the Krasnopolsky model (detailed in Tables 3-5) as well as the reaction rate. For the numbers, E9 is huge amounts, E8 is much, E7 is some, E6 is a little, E5 and below is “not so much”. The average altitude of greatest rate for the reaction is also shown. Remember that other reactions can be using up this intermediate as well, so this doesn’t necessarily mean that this is where the greatest production occurs. Generally the hardcore ion-neutral chemisty is way up high, while the gentle haze-surface-catalyzed radical association reactions occur down low.
These schemes and mechanisms will start with the hydrocarbons first, and the nitriles next. This will be somewhat organized from most prevalent, to least prevalent. But key intermediates will be introduced before their products. So if you want to see how an intermediate got made, scroll upwards. The ultimate start point for everything will be UV photons + CH4 or UV photons + N2.
First up is ethane (C2H6)...
Posted by: Bill Harris Jun 11 2010, 04:13 PM
Following each installment...
Posted by: rlorenz Jun 12 2010, 01:00 AM
Nice presentation.
It'd be interesting to add in some of the older models (Yung, Lara etc.)
btw it is possible that the number of significant digits displayed in your spreadsheet
exceeds the level of fidelity of the models, if not the attention span of the reader
Posted by: Juramike Jun 12 2010, 03:40 AM
Ethane (C2H6) [CH3CH3]
Almost all ethane is formed from the combination of two methyl radicals. The methyl radicals are initially generated in a whole bunch of ways previously described, a few are shown in the graphic above. The actual radical combination mechanism is not-so-straightforward, it is actually a three body reaction, with “M” in the reaction scheme designating an atom (or molecule) of a carrier gas.
What does this “M” do? I’m not exactly sure….clearly it comes out of the reaction as it enters it. It thus acts as a catalytic group or surface. My wild speculation is that it may help absorb (or impart) some of the kinetic energy required to make the reaction happen. The Krasnopolsky, 2009 Titan atmospheric model got this reaction rate and sequence from the Lavvas et al., 2008 Titan atmospheric model, who in turn modified it from Cody et al., 2003 (?) which was for Saturn/Neptune atmospheric modeling and had “M” = H2/He.
[In this case “M” is an inert gas. Confusingly, earlier ground state solution-phase chemistry literature (1961) showed that certain metal species (also labeled “M” in the literature reaction schemes) can help stabilize free radicals presumably by making an interaction between the metal and the radical. The additional stability helped the recombination reaction pathway, the yields were higher in the methyl radical combination to form ethane.]
Since this is a three-body gas-phase reaction, it is going to work much better in a more crowded environment lower in the atmosphere. It is very hard to get three molecules together in a rarified environment. This is one reason why many of the radical recombination reactions (most require a diluant gas “M” atom/molecule) will at lower altitudes.
Oddly enough, photodissociation of ethane to make ethyl radical is NOT the main way to form ethyl radical (C2H5.). Most ethyl radical will actually come from acetylene radical (HCC.) attacking ethane (C2H6). But we’ve got more mechanisms to describe before we get there..
Next up, the key intermediate ethylene (C2H4)...
Posted by: Juramike Jun 13 2010, 03:21 AM
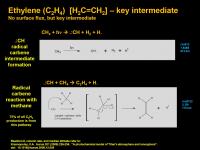
Ethylene (C2H4) [H2C=CH2]
Ethylene is a key intermediate that is a starting point for many pathways. If it's "inbound" is more than it's "outbound" there might be some that actually makes it down to the surface. But the Krasnopolsky 2009 model has a flux of zero - in that model it all gets used up.
The starting point is the generation of an extremely reactive intermediate - a radical carbene. This results from a high energy photon doing an almost total fraggo number on methane. The force of that photon blows out atomic hydrogen and a hydrogen radical. The molecular hydrogen gives a clue that this part was a concerted 2-electron process and so the carbene is spin paired and in a singlet state (as drawn in the diagram). This is in contrast to the other possible case where three hydrogen radicals get blown out and the carbene is spin unpaired and is in a triplet state.
The carbene can undergo a C-H insertion with a methane molecule (just wedging it's way between the C and the H) to make an ethyl radical transition state that probably only lasts a molecular vibration before kicking out a hydrogen radical and having both unpaired electrons dive into a double bond molecular pi-orbital. Et voila, ethylene is born!
While this process seems really funky, according to the Krasnopolsky 2009 model, 75% of all ethylene production follows this pathway.
Now that you've got ethylene, acetylene is only a UV photon away...
Posted by: Juramike Jun 14 2010, 02:52 AM
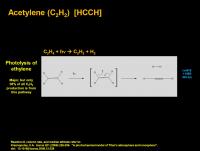
Acetylene (C2H2) [HCCH]
Acetylene has several formation pathways. The major route, although still only accounting for 38% of all acetyelene formation, is the photolytic dehydrogenation of ethylene, shown below:
Digging into serious detail, the way I understand this, the photon hits the pi-electron cloud and kicks it up to an excited state antibonding molecular orbital. This would be the LUMO (lowest unoccupied molecular orbital), which is now populated in this new singlet excited state. The electrons are still paired (singlet), they are just having nothing to do with each other (antibonding). While the pi-system molecular orbital is a bilobal fuzzy blob between the two carbon atoms, the antibonding molecular orbital faces away from the space between the two atoms. The spin paring is key to molecular hydrogen kicking out. All at once, the two electrons in both C-H bonds swap partners and you get molecular hydrogen and acetylene. Symmetry is preserved and all is harmonious.
If the two hydrogens came off as two radicals in one step, that would imply an initial singlet (most ground states are spin-paired) going to a triplet (spins-unpaired) state. This is a “forbidden” transition. It can still happen, it is just not normally allowed (and is thus rarer). It is also possible that the singlet excited ethylene could switch to a triplet excited ethylene, then kick out two hydrogen radicals. This switch is called “intersystem crossing” and constitutes a bonus step along the reaction pathway.
Posted by: Juramike Jun 15 2010, 02:58 AM
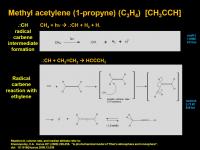
Methyl acetylene (1-propyne) (C3H4) [CH3CCH]
This compound is isomeric with allene (C3H4) [H2C=C=CH2].
It is created by initial formation of methyl radical carbene (same intermediate that reacts with methane to make ethylene), but this time, the radical carbene reacts with ethylene. I drew this as a carbene-style C-H insertion reaction where the inserted carbon atoms is also a radical. This can now kick out H radical and form a allene. This is just a 1,3-Hydrogen shift away from methylacetylene.
[EDIT: 6/16/2010: redid mechanism based on a C-H insertion route]
Posted by: jekbradbury Jun 15 2010, 07:35 PM
Isn't that allene, not cumene?
Also, I'm curious to know what mechanistic reason makes the cyclopropene ring preferentially decyclize to propyne instead of allene.
Posted by: Juramike Jun 15 2010, 10:39 PM
Whoops. You are right, it is allene not cumene. "Cumulene" (different spelling) is a generic term for compounds with two or more consecultive double bonds. Allene is the simplest of these.
Both allene and methylacetylene can exist in equilibrium. (equilibrium data here: http://en.wikipedia.org/wiki/Methylacetylene)
When I whipped this mechanism out, I woulda thought that the ring strain of the cyclopropene ring would be such that it would love to open up. However, now looking at "Carbenes, Nitrenes and Arenes" by T.L. Gilchrist and C.W. Rees, Appleton Century and Crofts, New York, 1969, I see that 1,1-dihalocyclopropenes are stable intermediates, and can be hydrolyzed to the corresponding cyclopropenone, at least if there are alkyl substituents on the cyclopropane double bond. (This results from a low yeild reaction of dihalocarbenes on a disubsituted acetylene).
Now with that little piece of information, I'd redo my proposed mechanism to have the radical carbene doing a C-H insertion, followed by radical jumping in and H. kicking out to initially form allene. Which then could equilibrates to methyl acetylene. Either way, you've got C3H4 - three carbon building block for further use....
Posted by: Juramike Jun 16 2010, 09:28 PM
[Well, whaddya know...cyclopropenone has been detected in interstellar space. It is especially stable because of the stability of cyclopropenyl cation. Presumably the oxygen in this molecule holds a partial negative charge to offset a partial positive charge in the cyclopropenyl part. See (freely available): http://iopscience.iop.org/0004-637X/642/2/933/pdf/0004-637X_642_2_933.pdf ]
Posted by: Juramike Jun 17 2010, 01:46 AM
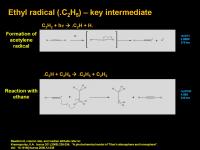
Ethyl radical (.C2H5) [.CH2CH3] - key intermediate
The direct photodissociation of a C-H bond in ethane is NOT the major process to ethyl radical. Instead, it is this:
The dominant process to ethyl radical is C-H photodissociation to make acetylene radical (.CCH), followed by it plucking a hydrogen radical from ethane to then generate ethyl radical. So in a sense it is an overall photodissociation of the ethane C-H bond, but it goes through an acetylene middleman.
Ethyl radical is important in the generation of propane, the fourth most abundant liquid on Titan. (after methane, ethane, and molecular nitrogen).
Posted by: Bill Harris Jun 17 2010, 02:47 AM
Mike, one thing I notice is that many of the reactions produce a hydrogen ion, an H+. A proton?? What eventually happens to this H+? If this will be answered in a later "installment", I'll curb my curiosity and wait...
--Bill
Posted by: Juramike Jun 17 2010, 03:03 AM
H+??
Dangit. Those should all be H. hydrogen radical.
I suspect this is a vector graphic-->jpeg issue. They look fine (i.e. little dots) in ACD (drawing package I'm using) and in Powerpoint. But now checking the Irfan view version they go from little dots to little plus signs. (Not cool.)
[Am I sure about this? Yeah, I'm positive! ![]() ]
]
Sorry about this, anyone got a fix?
Posted by: Bill Harris Jun 17 2010, 05:08 AM
Oh, H-radical as in "H-dot"? They look like awfully small "+" signs, so they are really dithered dots? I'd suggest trying to make them larger. But any sort of small dot in vector graphics is going to do that-- there is only so much you can do with 3pixelsx3pixels.
Sounds radical.
In the meantime, I'll revise how I view it... now it makes sense.
--Bill
Posted by: Juramike Jun 19 2010, 03:06 AM
Alright y'all, I'm real sorry about this, but I can't figure how to go from a Powerpoint slide over to a jpeg, png, or tiff without losing that little piece of resolution that turns a Powerpoint dot into a tiny little plus sign.
When in doubt, most of these equations should be with neutral radicals. (According to Krasnopolsky 2009 model, ion chemistry is important, but the neutral chemistry reactions (radicals, carbenes, radical carbenes) are still the driver.)
So if it looks like a tiny little plus sign, it should be a radical dot (.).
We soldier on....
Posted by: Juramike Jun 19 2010, 03:21 AM
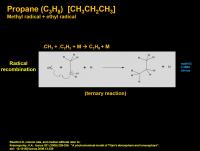
Propane (C3H8) [CH3CH2CH3]
The straightforward radical combination of methyl radical with ethyl radical (which originally came from acetylene radical reacting with ethane) gives propane. This happens with some of the atmospheric gas molecules acting as catalytic helpers.
Propane is the fourth most common liquid on Titan. (Methane, nitrogen, ethane>> propane).
[EDIT: Hah! I found a way to do the powerpoint-->jpeg without dither issues! First convert slides to pdf, then select and copy into Irfanview!]
Posted by: Juramike Jun 19 2010, 12:45 PM
Went back and fixed all diagrams in the different schemes. Radicals are radicals, and ions are ions. All electrons present and accounted for.
Posted by: Bill Harris Jun 20 2010, 07:18 PM
Looks good, Mike. Sorry to have tossed a monkey wrench with my "meatball chemistry", but it would have happened eventually.
Onward...
Posted by: Juramike Jun 22 2010, 10:59 PM
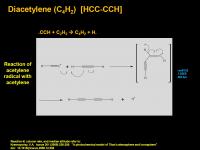
Diacetylene (C4H2) [HCC-CCH]
- also known as Butadiyne (locants not necessary)
Acetylene radical, formed by photodissociation of acetylene, attacks another molecule of acetylene. The transient radical intermediate kicks out a hydrogen radical to regenerate a triple bond.
And there you've got it, 4 carbon atoms joined up! This is one of the key intermediates that can go on to form benzene (C6H6) and even bigger stuff.
Interestingly enough, the formation of 1,3-butadiene goes by a totally different mechanism and different players.
Posted by: Juramike Jun 25 2010, 04:39 AM
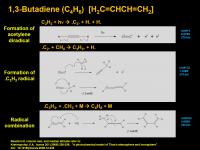
1,3-Butadiene (C4H6) [H2C=CHCHCH=CH2]
The formation of 1,3-butadiene starts with the double photodissociation of acetylene to give the acetylene diradical. (As drawn, with two H radicals coming off rather than H2, this would leave the diradical in a spin unpaired triplet state). This is pretty much just C2. One side of this high energy intermediate rips into methane and kicks out hydrogen radical to form a 1-propyne radical (one one radical of the original diradical gets "quenched"). This can then isomerize to allene radical, which is a key intermediate we'll see later (hint: benzene).
Allene radical can react with methyl radical to then form a methylated allene, which isomerizes to the 1,3-butadiene.
Posted by: PDP8E Jun 25 2010, 04:52 AM
Mike,
you had me at H2O
pdp8e ![]()
Posted by: Juramike Jun 26 2010, 04:57 AM
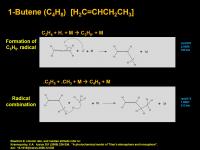
1-Butene (C4H8) [H2C=CHCH2CH3]
1-Butene is 1,3-butadiene's more saturated buddy. It has one double bond removed. To be fair, I'm not sure if this is the 1-butene isomer or the more thermodynamically preferred 2-butene, or the 2-methyl prop-1-ene isomer. (With four carbons, the iso-form is possible, it is the most stabilized double bond, and it makes the most sense mechanistically). Most likely, C4H8 would be a mixture of isomers. The mechanism will assume linear 1-butene is formed.
The sequence starts by the addition of hydrogen radical (atomic hydrogen) to allene to give allyl radical (C3H5). The middle carbon should be the best place to put the unpaired electron, but in order to form 1-butene, you need it on the end. Reaction of allyl radical with methyl radical at the C3 terminus gives 1-butene. The reaction of allyl radical with the unpaired electron at C2 (middle) should give the iso isomer - 2-methyl propene = isobutylene (not shown in the diagram). From 1-butene, a 1,3 H-shift would give more thermodynamically preferred 2-butene. However, 1-butene is perfectly stable at room temperatures on Earth. 2-butene can exist in two forms, cis and trans. In cis the two methyls are on the same side of the double bond, in trans they are opposite. Generally, the trans forms of a double bond is more stable. Both cis and trans forms of 2-butane are stable and separable at Earth room temperatures. Once formed they should also be stable at Titan temperatures. The formation mechanism should "lock in" the isomer. However, there is always the possibility that an excitation, or collision, or protonation/deprotonation with a superacid (like CH5+) can isomerize or switch the double bond geometry.
Next up is saturated n-butane.
Posted by: Juramike Jun 26 2010, 05:00 AM
you had me at H2O
pdp8e
I'm still drawing up one of the dominate ion-neutral routes to benzene and friends. It is beautifully complex.
Posted by: Juramike Jun 29 2010, 04:03 AM
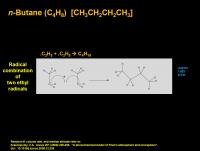
n-Butane (C4H10) [CH3CH2CH2CH3]
Boring butane comes from the straightforward recombination of two ethyl radicals (which comes primarily from acetylene radical ripping a proton off ethane to make ethyl radical). This happens primarily at very low altitudes.
In an atmosphere without a lot of acetylene photochemistry (H2 or methane-rich atmospheres), ethyl radical can form from C-H cleavage of ethane, but it is a lower efficiency process. So very small amounts of butane should be observed on Jupiter and the other H2 rich gas giants. (Recall that acetylene production requires .:CH, which is a 30% product of electronic recombination of CH3+ and an electron, and CH3+ doesn't form in H2 or methane-rich atmospheres, it forms best with Ar or N2 as a diluant gas, like on Titan)
Next up is the Mac Daddy of Titan's organics - benzene.
Posted by: Juramike Jun 30 2010, 03:56 AM
Benzene (C6H6)
At lower altitudes, benzene is formed from the combination of propargyl radical (.C3H3) This is a trimolecular event, it won’t happen very well in rarified environments – that goes by a different process.
The initiation is the formation of acetylene diradical and its reaction with methane to give .C3H3. When two of these (+some gas molecules) get together, they join, undergo 1,5 shift, cyclize, then undergo a final 1,3 H-shift to generate benzene.
Posted by: Juramike Jul 1 2010, 12:31 AM
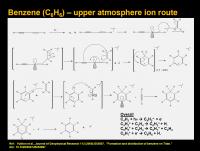
Benzene (C6H6) high altitude ion route
At higher altitudes, a different process occurs. This model is a little more speculative. The dominant proposed route starts with a large amount of diacetylene (C4H2) which gets photoionized to the radical cation. This then reacts with ethylene (by sucking in ethylene’s pi-electrons). The transient intermediate then kicks out atomic hydrogen (H.) and we are left with a C6 cation. This can cyclize via [3,3]-sigmatropic rearrangement, a concerted set of three two-electron processes. This gives us C6H5+. Reaction of this reactive intermediate with either molecular hydrogen (H2) or ethylene (C2H4) gives us protonated benzene (C6H7+) AKA the benzenium ion. If the reaction is with H2, H2 is sucked up into the system, if the reaction is with C2H4 (as drawn above) acetylene is spit out. The ethylene acts as a molecular hydrogen donor.
On electronic recombination, benzene is liberated. Normally, electron recombination is a pretty harsh process and it can frag up most molecular cations when they recombine. But benzene has many vibrational modes associated with it and so can sometimes get through this OK.
The speculative part is that the proposed Vuitton et al. 2009 literature model used a 10x higher amount of C4H2 than could be accounted for with the formation models for C4H2. Hopefully more measurements and theoretical work will help refine the formation models. But at least the model is consistent with Cassini observations of benzene abundance.
Posted by: Juramike Jul 2 2010, 12:46 AM
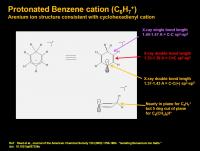
Structure of Benzenium (C6H7+)
This is one of the predominant ions in Titan’s upper atmosphere. It forms from protonation of neutral benzene, or by the reaction of C6H5 with a “hydrogen molecule” donor – either H2 or ethylene.
Recently, the structure of this cation was elucidated by X-ray crystallography by making a superacid salt. Up until recently, it wasn’t clear what the structure of this cation was, was the proton floating above the pi-system of benzene (a pi-complex) or was it localized to one of the edge carbons (a sigma-complex). Or would benzene have it’s normal pi-system, and enter into a 3c2e sp3 bond with two of it’s hydrogen atoms? The answer is more than academic, since the protonation and downstream reaction of benzene is an important process in the synthesis of many products and pharmaceuticals.
Based on the X-ray analysis, it looks like the proton formally latches on to one of the benzene carbons to make a sp3 hybridized CH2. The remaining pi-system (only 5 carbons now) locks up to a cyclohexadiene structure, with two formal double-bonds (sp2 hybridization), and a carbocation somewhat localized to the distal sp2-hybridized carbon from the CH2. While the benzenium molecule itself is flat, more substituted analogs will allow the CH2 to bend slightly out of plane, as you’d expect if that carbon was no longer part of a conjugated pi-system.
Posted by: Juramike Jul 4 2010, 01:46 AM
Beyond Benzene - PAH's and Polyphenyls
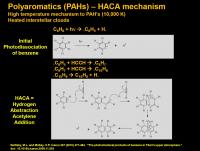
Polyaromatic hydrocarbons (fused aromatics)
Benzene is actually a pretty wussy molecule: it can cleave a C-H bond with “normal” UV light at 248 nm (= 115 kcal/mol). This generates a benzene radical (.C6H6) and a hydrogen radical.
One of the things benzene radical can do is react with acetylene (or diacetylene, or vinyl acetylene) and then close the pendant chain to form a new fused aromatic ring. This process is thought to be an important route to polyaromatic hydrocarbons during soot formation. The benzene radical first attacks the triple bond of acetylene, then places the radical at the terminal end of the alkyne that just got attached. This can repeat the process and react with another acetylene molecule. But this last radical now has a pendant radical that can attack the pi-system of the parent benzene ring to make a new six-membered ring. (Five and six-membered rings form pretty easily in most chemical processes). This places the radical likely at the ring junction (most substituted). This collapses to kick out a hydrogen radical and a fully aromatized fused ring system – naphthalene. Most studies indicate this can happen only at high temperatures – places like interstellar clouds illuminated with high energy photons or in the back of your car’s exhaust pipe.
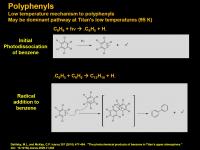
Polyphenyls (linked aromatics)
At lower temperatures, another process occurs with benzene radical, it reacts with another molecule of benzene. Attack of the radical into the pi-system gives a transient radical that quickly rearomatizes and kicks out a hydrogen radical. This creates a linked aromatic system (not fused).
Fused ring systems are usually planar. When they get really big, like C30 or so, they can become cup-shaped. Fused ring systems also can have different reactivities. In anthracene (3 benzenes fused in a line) the central carbons are very prone to oxidation. The central double bonds also are prone to reaction. (They do [4+2] cycloadditions easily). But as a general rule, fused ring carbons act more electron-deficient. PAH’s can do some funky chemistry.
In contrast, polyphenyls are about as exciting as linked benzene. The chemistry is almost exactly like that of benzene. Structurally, rings are twisted out of plane in the gas phase, but will flatten out when excited. In solid phase, the rings are planar. (All this is assuming that the hydrogens are at the ortho position. If there is a larger substituent, it will twist. For ortho-terphenyl, the two rings are oriented perpendicular to the central ring to prevent bumping each other.)
PAH’s require high temperatures to form, while polyphenyls can form at lower temperatures. It is likely that the low temperatures on Titan prefer the formation of polyphenyls such as biphenyl, terphenyl, and higher. There are many recent reports coming out that implicate the formation of biphenyls in Titan’s atmosphere.
Posted by: Bill Harris Jul 8 2010, 12:44 PM
The genesis of Titan's atmosphere may be more complex than we've ever imagined:
http://www.sciencedaily.com/releases/2010/07/100707002535.htm
--Bill
Posted by: Juramike Jul 13 2010, 12:00 AM
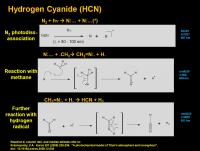
HCN
The incorporation of nitrogen into Titan’s organics usually results in the introduction of a nitrile group (-CN), where a terminal carbon atom is bound through a triple bond to a nitrogen atom. The nitrogen has a lone pair of electrons on it, although these may or may not be drawn in. (i.e. –CN:) This lone pair would love to donate to a proton (or other atoms desperate for electrons such as Lewis acids.)
The whole cascade starts with the blowing apart of molecular nitrogen by strong UV light. There are lots of ways this can happen, one is shown above. In this case, “lower energy” ultraviolet light in the 80 - 100 nm range (which is still pretty dang powerful) excites the molecular nitrogen to the point that it goes total fraggo and liberates a “naked” nitrogen atom and an “excited naked” nitrogen atom*. (The “excited naked” nitrogen atom will be a big player in tholin formation mechanisms). Other ways to get there include ionization of molecular nitrogen with light below 80 nm, then electron recombination back to molecular nitrogen, which is a pretty violent process, and then a total fraggo reaction that again generates a naked nitrogen atom and an excited naked nitrogen atom.
The “naked” nitrogen atom can react with a methyl radical to form a transient CH3N nitrene complex** (my guess is it would be likely in a triplet or an unpaired excited singlet state) that then blows out hydrogen radical to give H2CN. radical. This can react with another hydrogen radical to then kick out molecular hydrogen (H2) and HCN.
[I’m not sure why this process goes stepwise, I would think it possible for the transient CH3N carbene to kick out two hydrogen radicals (if triplet) or molecular hydrogen (possible if unpaired excited singlet state?) all in one go].
According to Krasnopolsky et al., 2009, this sequence accounts for 72% of all HCN formed in Titan’s atmosphere. But a recent article shows that excited state nitrogen chemistry may be also very important and poorly modeled. The atmospheric nitrogen chemistry of Titan is still poorly constrained, but getting better with recent lab experiments and further modeling. We’ll use the Krasnopolsky results, but these will likely shift on publication of the next model.
*****
*an asterisk [*] is used to designate an excited state atom. This is an atom where one of the electrons has been boosted to a higher energy orbital. Normally the electrons are spin paired in the orbital in the ground state. In an excited state atom the electrons can still be spin paired, but one of the electrons is now in a boosted energy orbital. So it may be in the unpaired excited state singlet state.
**very careful electron counting is important here, there are two lone pairs on nitrogen with a bonus electron, so naked nitrogen is like a triradical with a lone pair [:N...]
For the whole reaction we get: radical + radical nitrene (nitryne) --> radicals pair - nitrene (2 lone pairs on nitrogen) --> nitrene --> radical + radical
Posted by: Juramike Jul 14 2010, 12:11 AM
.CN radical - key intermediate
The formation of .CN: radical is very straightforward: Higher energy photon hits HCN, causes dissociation of H-C bond, and you get H. and .CN: radical.
.CN: indicates that the radical electron resides on the carbon atom, that the triple bond between carbon and nitrogen still exists, and that the lone pair is still on nitrogen (in an sp-orbital sticking straight out along the axis of the C-N triple bond.). Likewise the radical electron resides in the sp-orbital on the carbon and sticks straight out as well along the axis of the C-N bond away from the triple bond electrons. This molecule is linear, like acetylene.
.CN: radical can play a key role in the formation of cyanoacetylene [HCCCN], cyanogen [NC-CN], and dicyanoacetylene [NC-CC-CN], although there are alternative mechanisms for these compounds that proceed through cyanomethylene carbene (:CH(CN)).
Posted by: Juramike Jul 19 2010, 12:03 AM
Cyanomethlyene carbene [:CH(CN)]
A very recent article (June 2, 2010 issue of PNAS) by Imanaka and Smith has provided laboratory evidence that high energy photons can ionize nitrogen and cause it to incorporate it into Titan’s organics much more easily than previously thought.
The process is the initial photoionization of molecular nitrogen by EUV photons (wavelenghts < 60 nm) which then makes molecular nitrogen radical cation by blowing an electron out of the molecule. At some point, another electron recombines with the nitrogen molecule (cue dropping bomb sound) which releases a huge amount of energy. The energy released blows apart the dinitrogen triple bond and we are left with a “normal” nitrogen atom radical nitrene (nitryne) and an “excited” nitrogen atom radical nitrene (nitryne). (this is the stepwise sequence of the concerted sequence discussed for HCN – the rates of these steps were deliberately left of the graphic since the Imanaka and Smith work will definitely supercede the Krasnopolsky estimated models.)
The excited nitryne atom is likely in an excited state that accesses D-orbitals. These may be in unpaired spin-coupled state, but well run through the mechanisms assuming it acts like a triradical. (i.e. the actual process may be more concerted). In the case to make cyanomethylene carbene, the excited nitryne reacts with an alkyne. Drawing a stepwise mechanism, the first thing that happens is one of the pi-electrons of the triple bonds forms a new bond with one of the electrons of the nitryne. This now makes a carbon radical nitrene. (still three unpaired electrons in the system). A C-H bond breaks, and hydrogen radical (H.) goes away, and the remaining electron on carbon now forms a carbon-nitrogen double bond with one of the nitrene electrons.
A double-bond equilibration gives cyanomethylene carbene. But what does this molecule look like?
Spectroscopic data suggests that this molecule in the ground state is “close” to a linear molecule, with an H-C-(CN) bond close to 180 degrees That means that the likely hybridization on the “carbene” carbon is sp with the unpaired electrons in two p orbitals in a triplet state. (Ground state suggests it is in an unpaired spin-uncoupled triplet configuration, I’d guess on Titan that these molecules are likely in an excited unpaired but spin-coupled triplet configuration.) Why is this important? It’s probably academic, but it implies that many of the downstream steps could be concerted. This might become important if we deal with molecules (e.g. cis or trans double bonds) with stereochemistry, in this case the stereochemistry would be preserved. In a triplet stepwise reaction, there is always a chance for bond twisting before the second bond snaps shut, causing a loss of stereochemistry.
The :CH(CN) intermediate may be the key intermediate in the formation of tholins and for the incorporation of bonus* nitrogen into Titan’s organics.
It is also an intermediate that gives another route to many of Titan’s organics, such as cyanoacetylene, cyanogens, acrylonitrile, and ethylacetonitrile.
(*more that previously thought)
Posted by: GEmin Jul 19 2010, 06:36 AM
http://uanews.org/node/32574
Posted by: Juramike Jul 21 2010, 02:08 AM
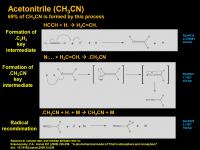
Acetonitrile (CH3CN)
There are multiple ways to form acetonitrile. One way is the addition of hydrogen radical to the triple bond of acetylene to generate the C2H3 radical. (vinyl radical, .CH=CH2). This can react with naked nitrogen (not clear if it needs to be excited or not) to generate a funky nitrene-enamine intermediate/transition state which can quickly kick out a hydrogen radical and the other electron combines with one of the free electrons on the nitrene to create an “iminoketene” radical. A quick tautomerization creates the CN triple bond and places the radical electron on the CH2 carbon. This can then find an Hydrogen radical floating around (maybe the very one that got kicked out a second ago) and then forms acetonitrile. According to the Krasnopolsky model, this mechanism using multiple transient intermediates accounts for 69% of the acetonitrile generation.
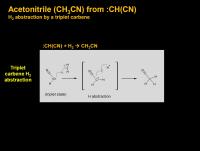
another possible way from :CH(CN)
Another way shown above is via hydrogen abstraction from our new best friend cyanomethylene carbene (:CH(CN)). Very low temperature studies(1) with carbenes have shown that triplet carbenes can react with molecular hydrogen, but that singlet carbenes cannot. (I’m not sure what an excited singlet carbene would do). These low temperature studies were done at VERY low temperatures, less than 30 K. This is waaay colder than Titan’s relatively balmy 95 K (or lower atmosphere minimum of 70 K). Interestingly, the authors found that while the overall formation of carbenes adding to H2 is exothermic, that there is a significant energy barrier to cross. Singet carbenes can’t do it. Singlet carbene insertion requires the carbene to concertedly (all-at-once) muscle in between the H-H bond. But triplets react a different way, they react like diradicals, one step at a time: the first step is an abstraction of one hydrogen atom from molecular hydrogen, then a combination of the two resulting radicals (the .CH2CN and the leftover H.). But even those transition state energies (IIRC +5 kcal/mol) are just a tad too high at 30 K to work, so the authors proposed quantum tunneling of the hydrogen radical. This is a bit out of my comfort zone, but the authors did detect the hydrogenated carbene products so this is experimentally valid. Also interestingly, molecular deuterium did NOT react. The energy barrier (and quantum tunneling barrier?) for a deuterium nucleus appears to be just too high in a 30 K matrix. The authors propose that the reaction with H2 can actually be use to as a mechanistic test as to whether a particular carbene is in a singlet or triplet state. If it hydrogenates, then it was in a triplet state.
So if the :CHCN formed photochemically high in Titan’s atmosphere is in a triplet state, it could react with molecular hydrogen to easily form CH3CN in one quick step.
This particular reaction was not modeled in the Krasnopolsky model, but I’d assume it should be in the next iteration.
:CH(CN) formed up in Titan's atmosphere is in a rarified environment and will be in a different environment than stuff in a low temperature inert matrix in a terrestrial laboratory. For one thing, the stuff in a matrix will be bumping around in it's cage and be able to relax to it's desired ground state. Not so for the stuff in Titan's upper atmosphere. That stuff is blasting along in a vaccum, all excited. If the first thing it bumps into and reacts with is H2, it can react as an excited species, which may not be in the ground state configuration. So the state of the :CH(CN) carbene will determine it's reactivity and propensity to form acetonitrile via direct hydrogenation.
Reference: (1) Zuev and Sheridan, Journal of the American Chemical Society 123 (2001) 12434-12435. "Low-Temperature Hydrogenation of Triplet Carbenes and Diradicaloid Biscarbenes - Electronic State Selectivity." doi: 10.1021/ja016826y]
Posted by: Juramike Jul 22 2010, 01:13 AM
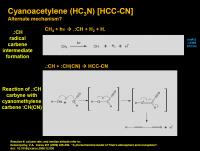
Cyanoacetylene (HC3N) [HCC-CN]
The dominant mechanism (according to the Krasnopolsky 2009 model) is the reaction of nitrile radical (.CN) with acetylene. After the initial addition into the triple bond, the C-H bond of the secondary carbon breaks homolytically, and the hydrogen radical leaves the system, while the newly unpaired electron on the carbon jumps into an empty molecular pi-orbital with the other unpaired electron on the carbon radical to form a triple bond. According to the mode, 62% of the cyanoacetylene is formed by this route.
However, there is another possible route to this molecule, again using our friend :CH(CN). In this case, photoionization of methane forms a high energy ionized intermediate CH3+ then undergoes electron recombination to violently produce .:CH. The Krasnopolsky model actually makes this occur in one step. Either way, you get methyne (the simplest carbyne) which then can react with methylene carbene. This could go a number of different ways, I’ve drawn the fully stepwise mechanism as if we were dealing with a triradical CH, and a diradical :CHCN. In the first step, a single bond is formed between the two carbon atoms. The resulting carbene-radical then combines to form a double bond to make a transient acrylonitrile radical. This kicks out a hydrogen radical (from the secondary carbon) as the resulting carbon radicals combine to form the triple bond (like the step above).
Posted by: Juramike Jul 23 2010, 12:17 PM
Cyanogen (C2N2) [NC-CN]
According to the Krasnopolsky 2009 model, cyanogen forms by two main routes. The first route (53% of cyanogen formation) is from the attack of nitrile (.CN) radical on acetonitrile radical (.CH2CN) which then kicks out methylene carbene and forms a new bond between the two nitrile carbons. [I’m baffled by this mechanism, why break a perfectly good C-C bond? You’d think the two radicals would combine to form malononitrile (propanedintrile (NCCH2CN) – a nice stable molecule and handy organic building block]
The other main route (47% of cyanogen formed on Titan) uses cyanomethylene carbene :CH(CN) and an excited naked nitrogen atom. (The top reaction in the scheme shows the formation of the cyanomethylene carbene from the reaction of excited naked nitrogen and acetylene). In this case, the :CH(CN) carbene reacts with another atom of excited naked nitrogen to form a radical carbene. This is shown as the fully stepwise mechanism, it is possible that some of these steps could be concerted. The next step is the radical carbon electron combining with one of the nitrogen nitrene electrons to form a C-N double bond, leaving an unpaired electron on the nitrogen – a nitrogen radical. The last step is a C-H bond hemolytic cleavage followed by the unpaired electrons, one from the nitrogen radical and one from the new carbon radical, jumping in together to form a C-N triple bond.
Posted by: Juramike Jul 25 2010, 04:04 PM
Dicyanoacetylene (C4N2) [NC-CC-CN]
Two different literature models have two different pathways to form dicyanoacetylene (C4H2). The route described in Krasnopolsky 2009 model has nitrile radical (.CN) attack the carbon-carbon triple bond of cyanoacetylene (HCC-CN). This makes an intermediate central double bond with one of the carbons holding an unpaired electron. Next door, the C-H bond cleaves, and hydrogen goes flying away as hydrogen radical (H.) and the resulting unpaired electron dives in with the carbon holding the unpaired electron and makes the central triple bond.
The Wilson and Atreya 2004 model proposes that two molecules of cyanomethylene carbene (:CH(CN)) connect their unpaired electons together to form a middle double bond. The resulting intermediate (1,2-dicyanoethylene), blows out H2 most likely in a concerted 4-electron pathway (2 sets of 2 paired electrons – this is a very typical concerted reaction, benzene resonances and [3,3]-sigmatropic rearrangements are 3 sets of 2 paired electrons (6 electron concerted pathways)) to give the final dicyanoacetylene and molecular hydrogen (H2).
Posted by: Juramike Jul 29 2010, 10:42 PM
Acrylonitrile (C2H3CN) [H2C=CHCN]
This molecule also has two routes according to the Krasnopolsky 2009 model. In the first route ethyl radical adds to the CN triple bond of cyanide to make a new C-C single bond and an intermediate radical on the nitrogen. The nitrogen radical electron dives in while a C-H bond on the other side homolytically cleaves. The two electrons reform a CN triple bond and the resultant hydrogen radical goes flying off.
The second route starts with cyanomethylene carbene (:CHCN). Reaction of this carbene with methyl radical (CH3) makes a new double bond as one of the electrons of the carbene (which may be in a triplet state) hooks up with the unpaired electron of the methyl radical. The resulting ethyl nitrile radical has the unpaired electron on the carbon alpha to the nitrile group. A C-H bond on the terminal carbon cleaves and one of the unpaired electrons joins the other unpaired electron to form a double bond. The other unpaired electron flies off with the hydrogen nucleus to be a hydrogen radical.
Acrylonitrile is known to polymerize when a suitable base is added. On Earth, it is a major industrial chemical product (several million tons/year scale) used for making all sorts of plastics and polymers. On Titan's surface, if a large concentration of this were present, and with a suffficient thermal “kick” it could undergo polymerization or further reactions in the presence of a base such as an organic amine. (Polymerization from initial nucleophilic Michael addition (1,4-addition) to the beta-carbon, then the resulting enolate undergoes Michael addition to another molecule of acrylonitirile, etc.)
Posted by: Juramike Aug 2 2010, 03:02 AM
I’ve tried to represent Titan’s chemistry in a slightly different way than is normally presented in the literature, the graphic below shows only the key intermediates that react with each other to form major hydrocarbon components in Titan’s atmosphere. Only the dominant pathway is shown for each molecule, so this is a more limited view than the typical “splat diagrams” (example splat diagram in http://www.unmannedspaceflight.com/index.php?s=&showtopic=6579&view=findpost&p=160771 this thread) shown for Titan chemistry:
On the left side of the matrix are key reactive species. Along the top are some “target” compounds and reactive intermediates. At the cross point are the species formed when these two meet. Note that some of the new species then go back up into the top row or left column. (Everything starts with nitrogen or methane).
In red on the left side is the highly reactive intermediate .:CH radical carbene. This is formed from the electronic recombination of CH3+, which itself was formed from N2 radical cation reacting with methane (the N2 radical cation formed from photoionization of nitrogen using EUV light.) All the compounds formed downstream from .:CH are also boxed in red. Note that almost all the unsaturated intermediates propagate from this compound. As stated before, if there was no CH3+, these couldn’t be formed, so atmospheres with large H2 components (like Jupiter and Saturn) would shunt away from the CH3+ pathways, and only the unboxed (boring saturated aliphatic) compounds would be formed. Think of the boxed compounds as Titan special menu items. This graphic may be valid on exoplanet atmospheres as well, boxed compounds show only those components that could exist where H2 (or other CH3+ absorbing) are significantly absent and allow CH3+ to frag itself up on recombination.
Posted by: Juramike Aug 22 2010, 08:06 PM
The graphic below shows the pattern of dominant reactions that give nitrogenated products in Titan's atmosphere. On the left side are key reactive species, and on the top are "target" species, some of them used to derive the reactive species.
Note that almost all products can be obtained using cyanomethylene carbene (:CH(CN)), although this may or may not be the dominant route.
Also note that almost all products ultimately come from unsaturated hydrocarbon chemistry, which themselves can only form in a relative absence of H2 (see above post.)
Only HCN would really be expected to form in a hydrogen-rich atmosphere. All the other pathways would be shut down due to CH5+ formation.
Posted by: Juramike Aug 25 2010, 01:41 AM
As requested (twice now) here is a comparison of some of the earlier pre-Cassini models with more recent atmospheric model production rates. Again normalized to Krasnopolsky et al., 2009:
(Done using conditional formatting in EXCEL, most literature models only have 2 significant figures listed. Extra digits displayed after normalization to set conditional formatting.)
Posted by: Juramike Aug 25 2010, 02:25 AM
Estimated depths solids vs. liquids from the various literature models:
Toublanc et al had a very large ethane flux (acetylene, too.). The Krasnopolsky estimated solids are higher due to the estimated amount of solid haze "C2H2/HCN copolymer" produced.
Posted by: Juramike Oct 8 2010, 02:26 PM
A recent DPS abstract discusses some possibilities for Titan chemistry:
Horst et al. DPS Meeting 42 (2010) Abstract 36.20 "Formation Of Amino Acids And Nucleotide Bases In A Titan Atmosphere Simulation Experiment".
Direct link to abstract http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=49dcd3e8-9c4d-4b9b-ac5b-e2f541f697e1&cKey=d7733270-c22a-4ab5-90b6-0605fc3d05ae&mKey={D515DFC0-245C-4047-81CC-C221DC1A54C6}.
One of the elements missing from all the above reactions is oxygen. There is not that much of it freely available in Titan's atmosphere. It is either in the form of H2O, from icy meteor infall, or from CO, or CO2. The 1 Gyr surface flux for CO2 varies between 10-100 cm for CO2 (high value in the Wilson and Atreya model), while the H2O surface flux (from meteors) is between 10 cm and lower (high value in the Raulin 1989 model). [A big Menrva crater splat may have caused actual water rain on Titan for a brief period according to an abstract a few years ago.]
The authors simulated Titan atmospheric conditions using N2, CH4, and CO2 and used a plasma discharge and generated molecules that incorporated oxygen. The abstract states that the following compounds were detected by GCMS (I'm assuming a direct injection of sample without a laboratory hydrolysis step before analysis.):
Amino acids glycine and alanine (presumably both enantiomers) were detected (but not H2NCH2CH2COOH?).
Also detected were the pyrimidine heterocycles cytosine, uracil, and thymine (but not orotic acid?)
As well as the fused heterocyclic imidazo-pyrimidine adenine (but not guanine?)
A slightly hyberbolic space.com article is http://www.space.com/scienceastronomy/saturn-moon-titan-atmosphere-life-ingredients-101007.html.
The key questions to relate this work are:
What was the overall yield of these compounds? Are we talking tiny trace amounts or significant geologically relevant surface deposits?
How doest this fit into the atmospheric models? Are these major pathways or minor chemical pathways?
How well does the PAMPRE experiment simulate Titan atmospheric tholins? (although PAMPRE is probably the best game in town for tholin simulation experiments)
How well does the simulated atmosphere reflect conditions in the upper atmosphere of Titan? At the critical formation zone? (Where is that for these pathways?)
And from a chemistry point of view, what are the electronic step-by-step mechanisms that make these?
Posted by: titanicrivers Oct 8 2010, 03:46 PM
Thanks for the discussion and the link Mike. One point that troubles a bit regarding Titan atmospheric simulation experiments is the lack of evidence so far for lightning on Titan. Is it safe to conclude that the sun's effect on the upper atmosphere of Titan is ionizing enough that lightning is not required to produce active species that can form into organic compounds. Do the plasma discharges and rf zaps of these experiments simulate the sun's effects or is there a presumption of lightning going on in Titan's troposphere that Cassini has just not detected as yet. (Hmm ... one wonders with the T72 storm whether the RPWS instrument lightning sensors were positioned to pick up a discharge?)
Posted by: Juramike Oct 8 2010, 05:21 PM
Most of the formation chemistry seems to be happening at very high altitudes, likely above any cloud-based lightning. (sprites?)
I think the laboratory experiments are trying to get molecular nitrogen to ionize, so either Extreme UltraViolet radiation (got beam source?) or plasma discharge are the best ways to do it. My simplistic mind views a plasma discharge experiment as a scaled up version of a mass spectrometer antechamber.
I'd suppose the major ionizing sources in Titan's upper atmosphere would be sunlight (very shortwave radiation). Not sure of the role that cosmic rays, energized particles (solar and trapped in Saturn orbit) or other electronic discharges would play.
Posted by: Juramike Jan 30 2011, 04:07 AM
LPSC 2011 abstract describes continuing efforts to identify heavier molecules from the Huygens GCMS using the flight spare at GSFC.
http://www.lpi.usra.edu/meetings/lpsc2011/pdf/1399.pdf
Why is this cool? Identifying heavier ions, and their relative amounts, can help constrain the atmospheric models and give a clue what's really down there on the surface.
A recent paper many of the same authors (Niemann et al. JGR (2010) E12006), showed that C2H6, C2H2, C2N2, and CO2 were detected at the surface after Huygens landed and volatilized materials in the muds. (Benzene (C6H6) was detected but couldn't be ruled out as atmospheric origin, maybe with more simulation analysis it can be confirmed?)
But even with mole fractions of these four components, if you normalize this and the atmospheric models to ethane, it can be seen that the Krasnopolsky model fits best for C2H2 and C2N2 (consistently 3x too low). The Cordier/Lavvas and Wilson and Atreya model have the predicted flux rate of C2N2 too low. See chart below:
(also attached as a spreadsheet):
Posted by: Juramike Feb 3 2011, 07:01 PM
Detection of high altitude cirrus clouds in Titan's atmosphere: http://www.nasa.gov/mission_pages/cassini/whycassini/titan-clouds.html
Posted by: centsworth_II Feb 9 2011, 04:32 PM
This gives hope of much greater understanding of Titan's atmosphere in the years to come.
http://www.nasa.gov/topics/technology/features/tofs.html
Located at NASA’s Ames Research Center, Moffett Field, Calif., this specialized facility, called the Cosmic Simulation Chamber (COSmIC)...
The chamber is the heart of the system. It recreates the extreme conditions that reign in space...
...Ames scientists delivered their first major milestone by coupling COSmIC with a cavity ringdown spectrometer, an extremely sensitive device that can detect the spectral fingerprint of matter at the molecular level.
Now, another major milestone has been achieved by coupling COSmIC with a time-of-flight mass spectrometer, an ultra-sensitive device that detects the mass of matter at the molecular level....
To understand Cassini’s data, scientists need this very powerful, very sensitive new tool. They will begin their analysis by forming molecules and species in the lab, measuring them in situ (inside their environment without disturbing them), and then trying to match their identity to Titan’s unknown aerosol molecules.
“Titan’s upper atmosphere data shows a rich spectrum. We will recreate those data in the lab and compare them to Cassini’s data. If they fit, great. If not, we will try something else. We will know when we are coming close to understanding them. We now have the right tool to do this,” said Salama.
Posted by: ngunn May 10 2011, 10:56 AM
Interesting link posted on the Cassini Huygens Yahoo group:
http://dx.doi.org/10.1038/ngeo1147
Posted by: Juramike Jul 3 2011, 12:55 AM
The Cool Way to PAH's in Titan's Upper Atmosphere
A recent article provides a new mechanism to get to fused ring systems at Titan-like temperatures and low pressures in the upper atmosphere. It is called EAM, or ethynyl addition mechanism. (The previously described HACA mechanism was adapted from high-temperature combustion studies).
The sequence starts with styrene, which can be formed by phenyl radical reacting with ethylene. This is known at high temperatures, but may or may not be valid at Titan upper atmosphere conditions. There may be an ion neutral chemistry route to styrene as well, but a a quick glance at the previous Titan atmospheric models, I didn't see styrene specifically mentioned.
Ethynyl radical (generated from EUV photodissociation of acetylene) attacks styrene at the ortho position to the vinyl substituent. The double bond opens, then the radical closes back in to kick out hydrogen radical, and you get vinylacetylenebenzene. This is almost barrierless and is pure downhill, so it can happen at very low temperatures.
[The authors calculated the collision rate at a simulated Titan upper atmosphere conditions, and found that the radiacal lifetime is shorter than any expected collision rates. It was estimated at 70 ms between molecular collisions at 90 K and 1E-6 mbar. So there is little chance ('bout 10%) another molecule can bump into the vinylacetylenebenzene and help remove all that excess energy. No relaxation, so the compound kicks out hydrogen radical. For those lucky molecules that do hit something, the benzene radical could become the final product of reactions and can then go on to do other things with other molecules...]
Another attack of acetylene radical, this time at the beta carbon (interior) of the alkyne substituent, generates a new radical that undergoes cyclization to form a bicyclic napthalene, but with a bonus alkyne substituent hanging off the end. It turns out this sequence is pretty much barrierless and downhill as well.
Why doesn't it form napthalene in the first sequence? It turns out that there is a pretty big activation energy barrier. So while that end product would be thermodynamically more downhill, the transition state energy mountain you need to go over is too big. So it shunts to the vinylacetylene benzene.
Here is a free link to the full paper. It is a pretty hardcore computational mechanism paper, but it does a great job of showing the snapshot-by-snapshot molecular movements and gyrations, including calculated energetics of the EAM process and alternatives that don't happen.
http://www.chem.hawaii.edu/Bil301/Kaiser%20Paper/p244.pdf
Posted by: scalbers Dec 11 2011, 08:39 PM
I saw a talk given at the AGU conference last week mentioning that far infra-red spectral observations suggest that the atmospheric hazes probably have different composition than the traditional Titan tholins. More lab work will be helpful in comparing various compositions to the observations. This is related to post #79 I believe. Here is the abstract...
ABSTRACT FINAL ID: P33F-0
TITLE: Spectral and vertical distribution properties of Titan’s particulates from thermal-IR CIRS data: Physical Implications
SESSION TYPE: Oral
SESSION TITLE: P33F. Titan: An Earth-Like World II
AUTHORS (FIRST NAME, LAST NAME): Carrie M Anderson1, Robert Samuelson2, 1, Sandrine Vinatier3
INSTITUTIONS (ALL): 1. Solar System Exploration Divis, NASA---GSFC, Greenbelt, MD, United States.
2. Astronomy, University of Maryland, College Park, MD, United States.
3. LESIA , Observatoire de Paris-Meudon, Meudon , France.
Title of Team:
ABSTRACT BODY: Analyses of far-IR spectra between 20 and 560 cm-1 (500 and 18 μm) recorded by the Cassini Composite Infrared Spectrometer (CIRS) yield the spectral dependence and the vertical distribution of Titan’s photochemical aerosol and stratospheric ice clouds. Below the stratopause (~300 km) the aerosol appears to be incompletely mixed for the following reasons: 1) the altitude dependence of the aerosol mass absorption coefficient is larger at higher altitudes than at lower altitudes, 2) the aerosol scale height varies with altitude, which implies some kind of layering effect, and 3) the aerosol abundance varies with latitude.
The spectral shape of the aerosol opacity appears to be independent in altitude and latitude below the stratopause, even though inhomogeneities in the abundance appear to be prevalent throughout this altitude region. This implies that aerosol chemistry is restricted to altitude regions above the stratopause, where pressures are less than ~0.1 mbar. The aerosol exhibits an extremely broad emission feature with a spectral peak at 140 cm-1 (71 μm), which is not evident in laboratory simulated Titan aerosols (tholin) that are created at pressures greater than 0.1 mbar.
A strong broad emission feature centered roughly around 160 cm-1 corresponds very closely to those found in nitrile ice spectra. This feature is pervasive throughout the region from high northern to high southern latitudes. The inference of nitrile ices is consistent with the highly restricted altitude ranges over which these features are observed, and appear to be dominated by HCN and HC3N. At low and moderate latitudes these clouds are observed to be located between 60 and 100 km, whereas at high northern latitudes during northern winter these clouds are observed at altitudes between 150 and 165 km. The ubiquitous nature of these nitrile ice clouds is inconsistent with a simple meridional circulation concept, suggesting that the true dynamical situation is more complex.
KEYWORDS: [6281] PLANETARY SCIENCES: SOLAR SYSTEM OBJECTS / Titan, [5405] PLANETARY SCIENCES: SOLID SURFACE PLANETS / Atmospheres, [5422] PLANETARY SCIENCES: SOLID SURFACE PLANETS / Ices.
(No Image Selected)
(No Table Selected)
SPONSOR NAME: Carrie Anderson
Additional Details
Previously Presented Material: Most of the material was published in Icarus 212, 762-778 in 2011.
Contact Details
CONTACT (NAME ONLY): Carrie Anderson
CONTACT (E-MAIL ONLY): carrie.m.anderson@nasa.gov
Posted by: Paolo Jan 5 2012, 10:51 AM
not sure this is the best topic to post it to, this interesting paper was published on today's Nature:
http://www.nature.com/nature/journal/v481/n7379/full/nature10666.html
Powered by Invision Power Board (http://www.invisionboard.com)
© Invision Power Services (http://www.invisionpower.com)